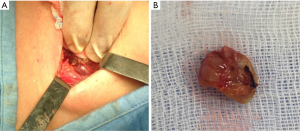
Management of undetectable and lost parathyroid adenoma
Introduction
The introduction of new technologies and the improvement of localization diagnostics have led to a change of strategy in the surgery of primary hyperparathyroidism (pHPT).
Focused approach to a preoperatively located parathyroid adenoma and the use of intraoperative parathyroid hormone (IOPTH) determination simplify and accelerate the surgical procedure and reduce morbidity.
Nevertheless, the success rate of approximately 95–99% in the initial intervention could not be improved by the new or further developed technologies (1,2).
The parathyroid adenoma, which cannot be found intraoperatively, is still a surgical problem today (2,3).
De facto, the case is even more challenging in case of surgical training of persistent or recurrent pHPT (Table 1).
Table 1
| Persistent primary HPT |
| Immediate post-operative hypercalcemia |
| Diagnosed up to 6 months following surgery |
| First operation failed to normalize serum level calcium |
| Recurrent primary HPT |
| Hypocalcemia or normalization for a time period at least 6 months |
| Hypercalcemia develops >6 months postop |
HPT, hyperparathyroidism.
Yen et al. reviewed a prospective database of consecutive patients with pHPT who underwent reparative pHPT with IOPTH monitoring (4). Thirty-nine patients underwent 43 reparative pHPT for persistent (79%), recurrent (21%) disease. All underwent ultrasonography and sestamibi imaging; 56% underwent additional imaging studies. Sensitivity of ultrasonography was 56%, sestamibi 53%, both studies 67%, computed tomography (CT) 48%, magnetic resonance imaging (MRI) 67%, and selective venous sampling (SVS) 50%. IOPTH monitoring predicted accurately cure in 100% and failure in 78%. At follow-up, 92% patients were normocalcemic (4). The authors proposed that ultrasonography and sestamibi studies should be done before all reparative pHPT; failure to localize should prompt sequential CT, MRI, and SVS until localization is achieved. IOPTH monitoring defines cure and is recommended for all reparative pHPT (4).
Advances in preoperative imaging and use of IOPTH levels are even more important for the approach to reoperative parathyroidectomy.
Diagnosis of persistent and recurrent pHPT is dependent on the quality and duration of follow-up, as well as the availability of biochemical data in centralized repositories (Tables 2,3). The consequence are the high cost to the patient of missed or inadequate initial operation, physical effects of remaining hyperparathyroid, additional time off work, potentially invasive localization testing, reoperative surgery, increased risk of complication and expense (4).
Table 2
| Regrowth of hyperplastic parathyroid tissue (especially in familial HPT) |
| Regrowth of autotransplanted parathyroid tissue |
| Recurrent or metastatic parathyroid carcinoma |
| Parathyromatosis |
HPT, hyperparathyroidism.
Table 3
| Failure to identify/remove parathyroid adenoma |
| Failure to identify/remove all adenomatous or hyperplastic parathyroid tissue |
| Inadequate subtotal resection in four-gland hyperplasia |
| Subtotal resection of a parathyroid adenoma |
| Residual or metastatic parathyroid carcinoma |
| Parathyromatosis |
| Failed percutaneous ablation technique |
HPT, hyperparathyroidism.
Outline
pHPT is the second most frequent indication for surgery for an endocrine surgeon, with an estimated incidence 27.7 per 100,000 population, estimated prevalence between 1 per 100 to 1 per 1,000 (1-4).
Success rate of initial operation is between 85–90%.
Five to ten percent will develop persistent or recurrent pHPT (1,5).
Eighty-five hundred patients suffer missed parathyroid adenoma in USA (1-7).
Re-operations for persistent or recurrent pHPT represent 12–20% of all parathyroid operations in referral centers (1-7).
The majority of parathyroid surgery (>70%) in USA is performed by unexperienced surgeons.
The success rate of initial operation for pHPT is 85–90% for experienced surgeons, 70% for unexperienced surgeons (<10 procedures/year) (1-7).
Experienced surgeons have perfect knowledge of parathyroid embryology, anatomy, size, site variation, the criteria of use of intra-operative parathyroid hormone measurement, judgment for surgical strategy.
The success rate for repeat parathyroidectomy is 80–90% experienced surgeons, 50% unexperienced surgeons.
In Sweden about 806 parathyroid operations/year are performed in 15 departments: first time operations are performed in 93% of patients, 60 parathyroid surgeries were reoperations (7%).
Most authors believe the true incidence of reoperative pHPT is higher (1-7).
De facto, little is known about what proportion of patients with persistent or recurrent pHPT undergo reoperation or the factors that influence the decision to proceed with remedial surgery.
Patients who have undergone non-parathyroid related surgery (i.e., total thyroidectomy) should be included in this challenging reoperative exploration group.
Cure rate of patients undergoing reoperation is lower than first operation 76.6% vs. 92.9%, P<0.0001 in univariate analysis and reoperation is associated with hypocalcemia P<0.0073 at logistic regression analysis (1-7).
Quality of life following reoperation may be impaired, especially following unsuccessful re-exploration or sternotomy, and this risk should be discussed with patients.
Risk factors
Patients with negative or inconclusive imaging more often have persistent or recurrent hypercalcemia (2,3,5).
Missed glands after parathyroidectomy for hyperparathyroidism can be found in standard locations in most cases.
No standardized set of objective criteria has been developed to classify parathyroid reoperations as preventable (Tables 4,5).
Table 4
| Re-do surgery |
| Coexisting large multinodular goiter |
| Coexisting thyroid malignancy |
| Thyroiditis |
| Supra-number of glands (>4) |
| Ectopic gland |
Table 5
| Inappropriate preoperative investigation |
| Inappropriate indication of surgery |
| Surgeon experience |
| Ectopic location |
| Occult double adenoma |
| Multi-gland disease |
Silberfein et al. in 2010 tried to categorize the locations of missed parathyroid glands found during reoperative parathyroidectomy and to determine any factors associated with these locations (8). The authors proposed a standardized nomenclature system based on the regional anatomy and the embryology of the parathyroid glands to guide a systematic exploration for parathyroid adenomas that are not easily identified and facilitate communication about gland locations. Fifty-four patients who underwent reoperative parathyroidectomy for persistent or recurrent hyperparathyroidism were included. Among 54 patients, 50 abnormal parathyroid glands were identified, resected, and classified as follows: 10% were type A (adherent to the posterior thyroid capsule); 22%, type B (behind the thyroid in the tracheoesophageal groove); 14%, type C (close to the clavicle in the prevertebral space); 6%, type D [directly over the recurrent laryngeal nerve (RLN)]; 18%, type E (easy to identify; near the inferior thyroid pole); 26%, type F (fallen into the thymus); and 4%, type G (gauche, within the thyroid gland) (8). No demographic, biochemical, or pathological factors were significantly associated with gland location (8). Among the 43 patients followed up for 6 months, 93% had documented cures (8).
One of the most common causes of failure is the occult second adenoma. Challenging of these conditions is: (I) second adenoma remains dormant or its function is suppressed until the first adenoma is excised; (II) rare identification on preoperative imaging; (III) not necessarily reflected in intraoperative intact parathyroid hormone (iPTH) measurement.
Furthermore, in pHPT the predictive value of technetium 99m sestamibi single emission computed tomography (Tc99m-MIBI-SPECT) for localizing pathological parathyroid glands before a first parathyroidectomy is 83–100%. Data are scarce in patients undergoing reoperative parathyroidectomy for persistent hyperparathyroidism (Table 6). A study was to determine the value of Tc99m-MIBI-SPECT in localizing residual hyperactive parathyroid tissue in patients with persistent pHPT after initial excision of one or more pathological glands (9). In patients with persistent pHPT, Tc99m-MIBI-SPECT accurately localized a pathological parathyroid gland in 33% of cases before reoperative parathyroidectomy, compared to 61% before first parathyroidectomy for sporadic pHPT. These data suggest that the accuracy of Tc99m-MIBI-SPECT in localizing residual hyperactive glands is significantly lower before reoperative parathyroidectomy for persistent pHPT than before initial surgery for sporadic pHPT (8).
Table 6
| Lower proportion of adenomas (44% vs. 74%) |
| Higher proportion of hyperplastic glands (55% vs. 26%) |
| More ectopically located glands (44% vs. 22%) |
| Smaller pathological gland size (1.21 vs. 2.03 cm) |
pHPT, primary hyperparathyroidism.
Failure to identify and remove a second abnormal gland in the case of double adenoma will probably become more common in the age of endoscopic surgery. Any attempt to limit the extent of the primary procedure will be insignificant if the risk of persistent or recurrent disease is increased.
The importance of the center/volume-outcome relationship has become increasingly clear in endocrine surgery (Table 7). Patients treated at high volume centers experienced durable euglycemia (8). The incidence of preventable operative failures in high- vs. low-volume centers after parathyroidectomy are significantly different 13% vs. 89%, where preventable operative failures were defined gland located in anatomical position, retroesophageal, intrathyroidal, thyrothymic ligament, gland resected through incision from the neck (6).
Table 7
| >50 cases/yr (Chen H. Ann Surg 2010) |
| >20 cases/yr (Mitchell J. Surgery 2008) |
| >10 cases/yr (Sosa JA. J Clin Endocrinol Metab 1998) |
yr, year.
Preoperative strategy
No randomized studies have been done to approach patients with undetectable adenoma or persistent or recurrent sporadic HPT. Current recommendations are based on case series and experts opinion (5,8-18).
Preoperative diagnosis
First reconfirm the diagnosis of hyperparathyroidism: the first and important measure to prevent unsuccessful parathyroid exploration is the correct diagnosis of hyperparathyroidism (Table 8).
Table 8
| Serum calcium (best ionized or albumin-corrected): increased |
| Parathyroid hormone: increased |
| Serum phosphate: decreased |
| Kidney scores: in the normal range (if elevated: elevated parathyroid hormone) |
| Vitamin D level: in the normal range (if lowered: elevated parathyroid hormone) |
| Alkaline phosphatase: indicator of increased bone remodeling |
| Calcium excretion in the urine: increased/normal |
An unequivocal confirmation of diagnosis of PHPT with definitive exclusion of other causes for hypercalcemia is obligatory (7).
Misdiagnoses are idiopathic hypercalciuria, malignancy and are reported in Table 8.
High parathyroid hormone levels at normal serum calcium levels rarely indicate normocalcemic hyperparathyroidism, but are almost always signs of vitamin D deficiency or renal insufficiency.
A high parathyroid hormone level and a high serum calcium level will clearly diagnose pHPT only if urinary calcium excretion is not decreased and the calcium clearance/creatinine clearance is >0.01, thus precluding familial hypocalciuric hypercalcaemia (FHH). FHH is an autosomal dominant hereditary disease caused by an inactivating mutation in the calcium sensing receptor. It is an important differential diagnosis of pHPT. The affected patients show an inadequate high parathyroid hormone, hypercalcaemia and hypocalciuria (5,7-18). For a calcium clearance/creatinine clearance ratio <0.01, the sensitivity for FHH is 85% and the specificity is 88%. A molecular genetic test confirms the diagnosis. The FHH usually requires no therapy. High serum calcium levels do not exclude FHH (16-18).
Diagnostic localization
Preoperative localization studies recommended before surgery combined both morphological and functional test improve localization rates with at least two positive and concordant localizing studies should be sought and available before reoperation too.
At least two positive and concordant localizing studies should be available before operation (or re-operation) to improve prospects of success, reduce operative time, and reduce operative morbidity.
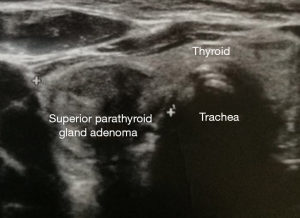
The most sensitive localization methods are ultrasound and 99mTc-MIBI (methoxyisobutylisonitrile) scintigraphy (Figure 1).
In a meta-analysis of 54 studies, sonography showed a sensitivity of 78.8% for solitary adenomas (8,11). Sonography fails in adenomas in ectopic position, in the anterior mediastinum or in the retro-esophageal area (8,11). The sensitivity of this procedure is usually higher, the larger the parathyroid gland is. In multi-gland diseases with lower: in a meta-analysis of 96 studies, the 99mTc-MIBI scintigraphy showed a sensitivity of 88.4% in solitary adenomas, but in multi glandular disease the sensitivity was only 44.2% (5,10-18).
With two positive localization diagnostics a “focused access” is possible.
The possibility of preoperative localization of a parathyroid gland has led to a change of procedure: in the case of positive localization diagnostics, a “focused access” to the suspected parathyroid adenoma is possible and classical bilateral exploration can be dispensed (19,20).
Persistent or recurrent disease
Surgeon in a multidisciplinary manner should review all documentations of the patient previously operated (16-18).
In case of persistent or recurrent disease the team should review the pre-/post-operative serum calcium and iPTH levels, all imaging or previous imaging, previous operative notes. Verify case of incomplete excision, complications, discrepancies (left vs. right, superior vs. inferior), intraoperative iPTH notes and the pathology report (21).
Still is scarce information available about CT, MRI or functional imaging (PET) as well as SVS in re-operative situation. The endocrine team should also employ complementary localization procedures, repeat sestamibi and high-frequency ultrasound, find for experienced nuclear medicine and radiologist, and include SPECT subtraction (22). Ultrasound-guided fine-needle aspiration with PTH measurement may be of value when abnormality is identified. If scintigraphy and ultrasound are inconclusive, MRI and/or CT are recommended. If scintigraphy, US, MRI, CT inconclusive, venous sampling for PTH is advised to indicate laterality. If sestamibi suggest abnormal mediastinal location, MRI/CT is indicated (23).
Venus sampling for PTH has a high sensitivity in re-operative cases (78–93%), indicates laterality and topography as neck or mediastinum. Venus sampling for PTH needs an experienced interventional radiologist for unusual venous drainage and aberrant anatomy secondary to the previous initial surgery (24).
Finally, pre-operative laryngeal examination is advised before re-do surgery.
Intraoperative management
The initial surgical approach, as bilateral or unilateral neck exploration, focused approach, minimally invasive radio guided parathyroidectomy will depend upon preoperative localization study findings, the presence or absence of thyroid enlargement and the experience of the surgeon (25,26).
Perfect knowledge of parathyroid anatomy and extensive experience of thyroid, parathyroid endocrine surgery is mandatory.
Possible reasons for the unsuccessful exploration are: (I) inexperienced surgeon; (II) the adenoma is in a typical position and the surgeon does not find it; (III) inexperienced nuclear medicine clinician; (IV) “false” ultrasound finding; (V) atypical adenoma; (VI) surgeon and nuclear medicine are experienced, but the adenoma cannot be found on the focused access (6,7,10,25,26).
The most common cause of unsuccessful parathyroid surgery is an untraceable adenoma in typical localization. Therefore, the exact knowledge of the topography of the parathyroid glands is indispensable. In case of coexisting nodular goiter, the parathyroid glands should be explored, if possible, in order to obtain a clear surgical site (26).
If there is no positive parathyroid adenoma in localization diagnostics, extended exploration (first unilateral, then bilateral) is indicated (6,7,10).
Karakas et al. found in 148 reparations 102 parathyroid glands in typical localization (69%), 15 in the thymus, 4 intrathyroid, 4 non-descended or in the carotid sheath, 8 retroesophageal, 8 mediastinal and 7 in the region of the aortic arch (13,14).
Reoperations
The multidisciplinary team must discuss extensively if reoperation is indicated. Current recommendations are based on case series and experts opinion and are (I) the same as for initial exploration; (II) patients <50 years old; (III) serum calcium level >11.5 mg/dL; (IV) serum iPTH >100 pg/mL; (V) symptomatic patients (Table 9) (5,8-18). Some experts do not recommend repeat surgery unless patient has significant symptoms and signs related to pHPT. However, patients who had unsuccessful initial surgery, have evidence of more severe signs and symptoms (25).
Table 9
| Indications are the same as for initial exploration |
| All patients <50 years old |
| Serum calcium level >11.5 mg/dL |
| Serum iPTH >100 pg/mL |
| Symptomatic patients |
iPTH, intact parathyroid hormone.
Possible contraindications to reoperative surgery include: (I) lack of certainty about the diagnosis of persistent and recurrent pHPT; (II) patients with minimal or equivocal symptoms and signs; (III) nonconvincing localization studies; (IV) comorbidity (Table 10).
Table 10
| Lack of certainty about the diagnosis of persistent and recurrent HPT |
| Patients with minimal or equivocal symptoms and signs |
| Non convincing localization studies |
| Comorbidity |
HPT, hyperparathyroidism.
Considerable disagreement continues about the treatment of patients with persistent or recurred mild asymptomatic hypercalcemia (7,9,12,22,25).
Definitive hypoparathyroidism can be a worse disease than mild asymptomatic persistent or recurrent pHPT (8,21).
Advanced age may predict against operation in the setting of recurrent disease (26). Elderly patients present specific features as comorbidity, unlikely to be referred for surgical treatment, unlikely to be referred to second chance of surgery, greater rates of postoperative complications, increased rate of multi glandular disease, kyphosis, loss of soft-tissue integrity, higher frequency of posterior located glands (2,5,6,22).
For patients who are not candidates to surgery because prohibitive medical comorbidity or refusing surgery, medical therapy or ablation under US guidance has been suggest, although hypercalcemia is likely to recur (26).
References for dissection
The search for an ectopic gland must be guided by the understanding of two mechanisms of ectopias: (I) congenital ectopias, i.e., abnormal embryology migration (“too little or too much”); (II) for acquired ectopias: migration affected by gravity or due to previous surgery (2,5,7,20).
Understanding of the embryology and surgical anatomy is essential (26).
The embryology of parathyroid glands explains the potential anatomical variants of locations. Surgeons performing operations must know all possibilities: 43% are superior parathyroid adenoma, 57% inferior parathyroid adenoma (2).
An ectopia cause: (I) failed operation; (II) increased morbidity; (III) more extensive dissection; (IV) scarring for a second operation (26).
Undescended parathyroid adenomas are defined and characterized as (I) glands located >1 cm to the superior thyroid gland; (II) not identified preoperatively; (III) mistaken for salivary glands on scintigraphy; (IV) difficult location for US.
Fallen parathyroid adenomas are defined and characterized by (I) thyrothymic ligament, thymic parenchyma location; (II) perithymic fat; (III) represent 9% of parathyroidectomies and 20% in autopsy studies.
In detail, the upper epithelial bodies develop from the 4th pharyngeal pouch and move in the embryonic phase towards the dorsal side of the upper thyroid pole. They are often pressed at the level of the cricoid cartilage flat against the dorsal surface of the upper thyroid pole. They are less variable in position than the lower ones. Eighty percent of the upper parathyroid glands are located approximately 1 cm cranial to the junction of RLN and inferior thyroid artery (10,19,26). Mobilization of the upper pole allows better exploration. The upper parathyroid glands can be palpated posterior to the thyroid gland (22,26). They are often located in the esophagotracheal groove or in the paraesophageal space, rarely retro-esophageal, where they can be palpated only after complete mobilization of the upper pole. The RLN is located ventrally to the upper parathyroid gland. Not infrequently, the upper parathyroid glands are also partially caudal to the inferior thyroid artery, but always posterior to the RLN. “Classical atypical” localizations are: para- and retro-esophageal, caudal to the inferior-thyroid artery paraesophageal, cranial to the upper thyroid pole. “Rare atypical” localizations are: posterior mediastinum, carotid vascular nerve sheath (15).
The more variable lower parathyroid glands are caudal to the inferior thyroid artery and of the RLN, usually in the region of the lower thyroid pole on the thyroid capsule, but often also slightly further from the thyroid gland, i.e., in the thyrothymic ligament, the connection between the lower thyroid pole and thymus horn (15,26).
The most “common atypical” location of the lower parathyroid glands is the upper thymus horn. Occasionally, a parathyroid gland located in the thymus horn can be found by digitally palpating the retrosternal space (26). The thymus horn is successively luxated ventrally.
Very rarely, the adenoma of the lower parathyroid gland is found above the inferior thyroid artery, and then corresponds to an undescended lower parathyroid gland. Evidence that this is the lower and not the upper parathyroid gland is the embedding in thymus tissue (15,18,26).
Helpful is the principle of symmetry: the parathyroid glands of one side are similar to those of the other side. If a normal parathyroid gland has already been visualized, the contralateral parathyroid gland is usually comparable (14). If, despite intensive exploration of all typical and atypical sites, no parathyroid adenoma has been found, the thyroid gland will be explored (26).
Double adenomas
Overall incidence is 3–15%, one of the most common causes of failure of surgery. Is synchronous or metachronous, i.e., this second adenoma remains dormant or its function is suppressed until the first adenoma is excised. Is rare the identification on preoperative imaging (sestamibi and US suggest double adenomas in 20–28% cases) and not necessarily reflected in intraoperative iPTH measurement. The pattern of anatomic distribution is interestingly 40–82% crossed bilateral configuration (4).
Intrathyroid parathyroid glands
“True” intrathyroid parathyroid glands are a rarity, usually “intrathyroid” parathyroid glands are not completely intrathyroid, but are only partially surrounded by thyroid tissue. Hyperfunctioning intrathyroid parathyroid gland presents an incidence 0–3.5% (19). Pre- or intra-operative US is most informative imaging method with >65% visible at thyroid surface gland. Incision of thyroid capsule gland and subsequent excision is usually advisable, rarely thyroid lobectomy (14).
Supernumerary parathyroid adenoma
If four normal parathyroid glands could be visualized, this may be a rare case of supernumerary parathyroid adenoma. Henry et al. found 9 ectopic parathyroid adenomas in a fifth gland (6 adenomas in the anterior mediastinum, 3 in the vascular-nerve sheath) in 1,307 patients with pHPT (12).
Intraoperative parathormone determination
If an intraoperative determination of parathyroid hormone serum levels is possible, it is advisable to have a lateral separation of parathyroid hormone from the jugular vein after the first unsuccessful bilateral exploration (10,26).
If no difference in side is measured, there are two possible causes: (I) the vein of the adenoma drains caudally into the jugular vein, then it is most likely a lower parathyroid adenoma or a “deep” upper; or (II) a multiple gland disease, either four enlarged parathyroid glands or two adenomas right and left (26). If there is a side difference, an upper parathyroid adenoma is more likely (11,26).
If the possibility of an IOPTH determination does not exist, it is advisable to remove blood from the jugular vein in order to determine the parathyroid hormone on both sides of the jugular vein before the end of the unsuccessful operation, in order to obtain an indication of the lateral location postoperatively (9,26).
Importance of localization diagnostics
Ultrasound is definitively a useful device with an experienced sonographer.
Ultrasound is also essential for the surgeon to improve location, precise incision and determine minimally invasive approach (2).
99mTc-MIBI-scintigraphy should be performed in experienced centers as well (26). Sestamibi is usually the recommended first test and include early (10–15 min) and delayed acquisition (3–4 h), includes right, left, anterior oblique views, pinhole collimator and pertechnetate subtraction to eliminate coexisting thyroid pathology (26).
For two matching localization procedures (sonography and scintigraphy), the probability of finding the adenoma at this location is 92% (16).
The surgery can then be scheduled as a focused surgery without the need for intraoperative parathyroid determination to control success (26).
With only one positive localization procedure, intraoperative parathyroid determination must be possible with planned focused access (26). If the adenoma is not found then it is explored bilaterally.
Multiple gland disease or a double adenoma should always be considered in negative localization diagnostics (12,15,26).
Data from the “Scandinavian Quality Register for Thyroid and Parathyroid Surgery” showed a negative exploration rate of 13.3% in 155 patients with negative preoperative localization diagnostics (3).
Strategy for unfindable parathyroid adenoma in upper or lower position
Upper parathyroid adenoma
It is necessary to fully mobilize the thyroid lobe medially, for better exposure with accurate hemostasis and fine dissection. Adenoma is usually placed deep in the RLN. Given intimate contact with the RLN, its continuous and routine exposure with the use of intraoperative neural monitoring (IONM) is advisable (Figure 2).
The exploration is carried out first para-esophageal, then to the posterior mediastinum.
In negative findings, the exploration is then extended to the retro-esophageal space, cranially to the upper thyroid pole and then the vascular carotid sheath (11,26).
Furthermore, the upper thyroid pole is prepared as well. Careful identification of the external breach of the superior laryngeal nerve is suggested.
If the findings are still negative, the thyroid gland is explored and resected in case of suspicious palpation or ultrasound findings and examined (26).
Lower parathyroid adenoma
After exploration of the lower thyroid pole, the thyroid ligament is dissected free and the thymus horn is successively luxated ventrally (2,7,26). Subsequently, an undescended lower parathyroid gland is searched for. This is usually embedded in thymus tissue. Since there is no evidence that intrathyroid parathyroid glands always correspond to the upper parathyroid glands, a thyroid resection is indicated here also in case of abnormal findings in the sonography or palpation. Contraindicated, however, is the sternotomy without clear proof of localization of a mediastinal adenoma (26).
Remedial surgery
Most missed parathyroid adenomas during re-operation for pHPT are found in normal anatomical location (1,6,7,20).
In missed parathyroid adenoma with unsuccessful neck exploration, 7–76% missed parathyroid adenomas during re-operation for pHPT are found in normal anatomical location. Reasons are usually: (I) the initial surgery not have understood anatomy; (II) initial surgery not able to explore the neck properly; (III) use of nonstandard terminology (normal vs. ectopic); (IV) another cause of persistent pHPT is failure to locate and remove an ectopic adenoma (2,7).
In recent years, inadequate resection of multi-glandular disease has become more common cause of persistent and recurrent pHPT (9).
The goal of re-operative surgery is to excise the abnormal parathyroid gland(s) and limit exploration to help minimize potential complications.
Pre- and post-operative laryngeal examination is mandatory (7).
Surgeon may choose an appropriate time and operative approach for remedial surgery in a previous lost parathyroid adenoma. It is suggested to perform surgery first week or after 3 months post-operative (for scar formation). For reduction of risk for RLN and remaining parathyroid gland, still a focused exploration is suggested, the “back door” approaches, the use of intraoperative determination of iPTH and use of IONM (Figure 3).
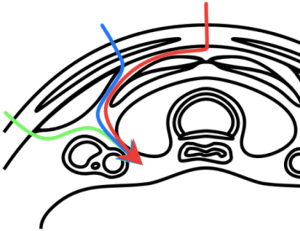
Intraoperative adjuncts for re-operative parathyroid surgery are: (I) use of iPTH is recommended; (II) radio-guided parathyroidectomy may be useful; (III) frozen section may be of value; (IV) methylene blue not supported; (V) Judicious use of parathyroid autotransplantation is recommended; (VI) continuous IONM may be of value (Table 11).
Table 11
| Use of iPTH is recommended |
| Radio-guided parathyroidectomy may be useful |
| Frozen section may be of value |
| Methylene blue not supported |
| Judicious use of parathyroid autotransplantation is recommended |
| IONM may be of value |
iPTH, intact parathyroid hormone; IONM, intraoperative neural monitoring.
The value of iPTH in reparative surgery is: (I) iPTH can lateralize hyperfunctioning parathyroid tissue with internal jugular vein sampling when pre-operative localization is uncertain or predict cure; (II) reduce need for continued exploration in the scarred neck; (III) permit ex vivo aspiration of excised tissue (Table 12).
Table 12
| iPTH is recommended (level III, grade B) |
| iPTH can lateralize hyperfunctioning parathyroid tissue with internal jugular vein sampling when pre-operative localization is uncertain or predict cure |
| Reduce need for continued exploration in the scarred neck |
| Permit ex vivo aspiration of excised tissue |
iPTH, intact parathyroid hormone.
Table 13 depicts which physiologic decrease of iPTH, and different criteria for iPTH drop used currently.
Table 13
| Successful criterion | Definition | Clinical evaluation advantages | Clinical evaluation disadvantages |
|---|---|---|---|
| Miami | IOPTH decrease >50% from most precise value 10 min after PTX | Good “all-rounder” ideal benefit/risk balance for suspected SGD in MGD | A little uncertain in MGD |
| Halle | IOPTH decrease up to half of the upper IOPTH standard value (≤35 pg/mL) 15 min after PTX | Very reliable exclusion of MGD | Unfavorable on manipulation, “slowly normalizer” in MGD |
| Vienna | IOPTH decrease ≥50% of the basal value 10 min after PTX | Good “all-rounder” | Unfavorable on manipulation, “slowly normalizer” in MGD |
| Ann Arbor | IOPTH decrease ≥50% of the basal value or manipulation value reaching the standard value range (12–75 pg/mL) 5 or 10 min after PTX | Good exclusion of MGD | Slightly variable without defining clear preference criteria unfavorable on manipulation, “slowly normalizer” in MGD |
| Rome | IOPTH value ≤35 pg/mL or IOPTH ≥90% from the basal value | Very reliable exclusion of MGD | Slightly variable without defining clear preference criteria unfavorable on manipulation, “slowly normalizer” in MGD |
| Aarhus | IOPTH value ≤20% of the basal value or reaching the standard value 5 min after PTX | Early criterion, low waiting time | Unfavorable on manipulation, “slowly normalizer” in MGD |
| Rotterdam | IOPTH value 100–200 ng/L and IOPTH decrease ≥70% 10 min after PTX or IOPTH decrease ≥200 ng/L and ≥80% 10 min after PTX | Adaptable with different kinetics | Variable definition with no clear preference criteria, too complicated |
iPTH, intact parathyroid hormone; IOPTH, intraoperative parathyroid hormone; PTX, parathyroidectomy; SGD, single gland disease; MGD, multiple gland disease.
Parathyromatosis
Rare indication for remedial parathyroidectomy is parathyromatosis. Parathyromatosis is the local seeding of parathyroid adenoma tissue as a consequence of initial surgery, usually from inappropriate manipulation and rupture of parathyroid gland. The fissure of the adenoma capsule cause spilled tumor cells that consequently grew in the tissue of the neck (16).
Sources of error and cost
The monetary and personal costs of unsuccessful parathyroid surgery were investigated (27). Total costs of reoperative parathyroid surgery were more than twice the cost of an initial operation (median, $8,383 vs. $3,948, P<0.001) because of the cost of radiologic studies (median, $3,378 vs. $43, P<0.001) (27). The cost to the patient of an inadequate initial operation includes the physical effects of remaining hyperparathyroid, additional time off work, potentially invasive localization testing, reoperative surgery with increased risk of complications, and substantial expense (27).
Conclusions
Regionalization of parathyroid surgery
Surgical volume influences the etiology of operative failures. Less experience surgeons more likely miss an abnormal parathyroid gland in normal anatomic location. Low volume centers have lack of experience, diminished technical skills, lack of familiarity with current standards of care.
Lack of localization may be a reasonable criterion to base referral of the patient to high volume endocrine surgery unit.
Since complication rates are higher and surgical cure rates are lower in reoperative surgery, employ extensive localization studies prior to reoperation and refer patient to high volume center.
Prospective
Despite the availability of expert surgeons and preoperative imaging investigations, some patients require re-operation for persistent or recurrent hyperparathyroidism.
The nature of preventable errors in parathyroid first surgery and reoperation are (I) technical, i.e., surgeon was in the correct spot of the neck but did not find the parathyroid; (II) judgment, i.e., surgeon fails to recognize clues for multi glandular disease; (III) victim, i.e., non-accurate preoperative imaging studies.
The opportunity for the development of practice guidelines regarding selection of patients for remedial surgery, given the greater threshold for operative intervention in high-risk population will be useful in the near future.
Guidelines may include common algorithm for optimal management of reoperative cases, definitive nomenclature to classify parathyroid location, multidisciplinary assessment between surgeons, radiologist, pathologist, etc., surgical planning (incision, patient positioning, type of anesthesia, duration, dissection, etc.).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2017.12.02). Gianlorenzo Dionigi serves as an unpaid editorial board member of Annals of Thyroid from Mar 2017 to Feb 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institution’s Ethical Board Committee approved the review. Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery 1984;95:14-21. [PubMed]
- Bergenfelz AO, Hellmann P, Harrison B, et al. Positional statement of the European Society of Endocrine Surgeons (ESES) on modern techniques in pHPT surgery. Langenbecks Arch Surg 2009;394:761-4. [Crossref] [PubMed]
- Bergenfelz AO, Wallin G, Jansson S, et al. Results of surgery for sporadic primary hyperparathyroidism in patients with preoperatively negative sestamibi scintigraphy and ultrasound. Langenbecks Arch Surg 2011;396:83-90. [Crossref] [PubMed]
- Yen TW, Wang TS, Doffek KM, et al. Reoperative parathyroidectomy: an algorithm for imaging and monitoring of intraoperative parathyroid hormone levels that results in a successful focused approach. Surgery 2008;144:611-9; discussion 619-21. [Crossref] [PubMed]
- Dotzenrath C, Cupisti K. Nebenschilddrüse in Viszeralchirurgie. In: Becker H (Hrsg). editor. Visceralchirurgie. München: Elsevier, 2000.
- Chen H, Wang TS, Yen TW, et al. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg 2010;252:691-5. [PubMed]
- Cheung K, Wang TS, Farrokhyar F, et al. A Meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol 2012;19:577-83. [Crossref] [PubMed]
- Silberfein EJ, Bao R, Lopez A, et al. Reoperative parathyroidectomy: location of missed glands based on a contemporary nomenclature system. Arch Surg 2010;145:1065-8. [Crossref] [PubMed]
- Witteveen JE, Kievit J, Stokkel MP, et al. Limitations of Tc99m-MIBI-SPECT imaging scans in persistent primary hyperparathyroidism. World J Surg 2011;35:128-39. [Crossref] [PubMed]
- Fraker DL, Harsono H, Lewis R. Minimally invasive parathyroidectomy: benefits and requirements of localization, diagnosis, and intraoperative PTH monitoring. long-term results. World J Surg 2009;33:2256-65. [Crossref] [PubMed]
- Fraser WD. Hyperparathyroidism. Lancet 2009;374:145-58. [Crossref] [PubMed]
- Henry JF, Defechereux T, Raffaelli M, et al. Supernumerary ectopic hyperfunctioning gland: a potential pitfall in surgery for sporadic primary hyperparathyroidism. Ann Chir 2000;125:247-52. [Crossref] [PubMed]
- Karakas E, Müller HH, Schlosshauer T, et al. Reoperations for primary hyperparathyroidism – improvement of outcome over two decades. Langenbecks Arch Surg 2013;398:99-106. [Crossref] [PubMed]
- Karakas E, Schneider R, Rothmund M, et al. Initial surgery for benign primary hyperparathyroidism. An analysis of 1300 patients in a teaching hospital. World J Surg 2014;38:2011-8. [Crossref] [PubMed]
- Nawrot I, Chudzinski W, Ciacka T, et al. Reoperations for persistent or recurrent primary hyperparathyroidism: retrospective Cohort study at a tertiary referral center. Med Sci Monit 2014;20:1604-12. [Crossref] [PubMed]
- Mihai R, Simon D, Hellman P. Imaging for primary hyperparathyroidism—an evidence-based analysis. Langenbecks Arch Surg 2009;394:765-84. [Crossref] [PubMed]
- Mihai R, Barczynski M, Iacobone M, et al. Surgical strategy for sporadic primary hyperparathyroidism an evidence-based approach to surgical strategy, patient selection, surgical access, and reoperations. Langenbecks Arch Surg 2009;394:785-98. [Crossref] [PubMed]
- Stechman MJ, Weisters M, Gleeson FV, et al. Parathyroidectomy is safe and improves symptoms in elderly patients with primary hyperparathyroidism (PHPT). Clin Endocrinol (Oxf) 2009;71:787-91. [Crossref] [PubMed]
- Raue F, Haag C, Schulze E, et al. Familiäre hypokalziurische Hyperkalzämie – aktuelle Diagnostik und Therapie. J Miner Stoffwechs 2009;16:80-3.
- Rothmund M. Clinical dilemma: A parathyroid adenoma cannot be found during neck exploration of a patient with presumed primary hyperparathyroidism. How should this problem be tackled? Br J Surg 1999;86:725-6. [Crossref] [PubMed]
- Rothmund M. Chirurgische Anatomie der Nebenschilddrüsen. In: Siewert JR, Rothmund M, Schumpelick V (Hrsg). Endokrine Chirurgie, 3. Aufl. Berlin: Springer, 2007: S235-42.
- Smith N, Magnuson JS, Vidrine DM, et al. Minimally invasive parathyroidectomy: use of ioPTH assay after 2 preop. localization studies. Arch Otolaryngol Head Neck Surg 2009;135:1108-11. [Crossref] [PubMed]
- Wachtel H, Bartlett EK, Keiz RR, et al. Primary hyperparathyroidism with negative imaging: a significant clinical problem. Ann Surg 2014;260:474-80; discussion 480-2. [Crossref] [PubMed]
- Wang CA. Parathyroid re-exploration. A clinical and pathological study of 112 cases. Ann Surg 1977;186:140-5. [Crossref] [PubMed]
- Wirowski D, Goretzki PE, Schwarz K, et al. Failed surgery in primary hyperparathyroidism – what has changed with time. Exp Clin Endocrinol Diabetes 2013;121:323-8. [Crossref] [PubMed]
- Dotzenrath C. Intraoperative management of undetectable parathyroid adenoma. Chirurg 2015;86:24-8. [Crossref] [PubMed]
- Doherty GM, Weber B, Norton JA. Cost of unsuccessful surgery for primary hyperparathyroidism. Surgery 1994;116:954-7; discussion 957-8. [PubMed]
Cite this article as: Catalfamo A, Famà F, Pergolizzi FP, Bartolo V, Rizzo AG, Marullo M, Fabiano V, Cancellieri A, Melita G, Portinari M, Donatini G, Makay O, Carcoforo P, Dionigi G. Management of undetectable and lost parathyroid adenoma. Ann Thyroid 2018;3:1.