
Neural monitoring in transoral endoscopic thyroidectomy
Introduction
In terms of safety and feasibility, transoral endoscopic thyroidectomy (TOET) is now considered comparable to other well established endoscopic thyroid procedures (1-3). However, notable advantages of TOET are its superior cosmetic outcomes (i.e., absence of scarring) (3,4). The accumulating literature on TOET reveals an expanding range of applications (5,6).
Recurrent laryngeal nerve (RLN) palsy remains a common morbidity after thyroid surgeries (7,8). The application of intraoperative neural monitoring (IONM) during thyroidectomy has gained increasing acceptance among surgeons and medical societies as an adjunct method of verifying functional integrity of the RLN (8-13). Although studies show that IONM increases the safety of endoscopic procedures (14-16), data for the use of IONM specifically in TOET are rarely reported. Therefore, the aim of this study was to review the current literature on the use of IONM in TOET.
Neural monitoring in endoscopic thyroidectomy
The first report on IONM in minimally invasive surgery was published in 2007 (15). In 2017, Dionigi et al. (17) performed a systematic review of literature relevant to endoscopic thyroidectomy for years 2000–2015. Of 160 reports of thyroidectomy retrieved in a literature search using evidence-based criteria, only nine (5%) reported the use of IONM. Eight studies reported the use of IONM for monitoring 522 nerves at risk. Only three were prospective randomized studies. The studies that reported the use of IONM endoscopic and robotic procedures included their use for re-surgery and their use in both benign and malignant cases. None of the IONM endoscopic procedures discussed in these studies involved bilateral palsy. Two studies reported the use of a staged strategy. The rates of transient and permanent RLN palsy were 0–3.6% and 0–0.4%, respectively. Only 30% of the studies performed vagus nerve (VN) stimulation, and only 25% performed superior laryngeal nerve monitoring.
Although endoscopy is routinely used for RLN identification, RLN palsy still occurs, even when IONM is used to elucidate the mechanism of RLN injury. For example, one study reported R2 and V2 loss of signal (LOS) in 6.9% (14 of 201) nerves at risk examined with IONM in video-assisted thyroidectomy (16). Notably, 80% of the lesions were located the distal 1 cm of the course of the RLN. The incidences of type 1 (segmental) and 2 (diffuse) RLN injuries were 71% and 29%, respectively. The most common injuries were traction injuries (70%) and thermal injuries (30%).
The use of IONM in transoral approach was first reported in 2009 (18). Ten bilateral thyroidectomies were performed by sublingual transoral access in a porcine model, and a percutaneous stimulating probe was used to record R1 and R2. In 2016, Wang et al. (6) used IONM to perform TOET via vestibular approach (TOETVA) with central neck dissection in ten thyroid carcinoma patients. A standard procedure for recording V1, R1, R2 and V2 was performed after the nerve monitoring probe was used to puncture the skin in the neck. No patients suffered transient or permanent RLN palsy. Inabnet et al. (19) video recorded the use of IONM in TOETVA. Laparoscopic Maryland dissecting forceps were used as the stimulating probe, and right lobectomy was performed with recordings of R1 and R2. Recently, Chen et al. (20) reported their preliminary experience in placing stimulation electrodes used for VN monitoring during continuous IONM in TOETVA performed in 20 patients. They concluded that continuous IONM is feasible and safe to use in TOETVA and can help prevent RLN palsy through early detection of adverse electromyography (EMG) changes.
Setting and standards for neural monitoring during TOET
In 2011, the International Neural Monitoring Study Group (INMSG) proposed guidelines for using IONM in thyroid and parathyroid surgery (21). The guidelines included standard procedures for performing equipment setup, endotracheal tube placement, intraoperative evaluation of LOS, and intraoperative troubleshooting algorithms. In addition to standardizing the use of IONM and the reporting of IONM results, the INSMG guidelines clarify the limitations of IONM and identify areas that require further research.
However, although the INMSG guidelines have established standard procedures for using IONM in thyroidectomy, no guidelines have been established for using IONM in TOET. Therefore, this study proposes the following standards
Team
A skilled anesthesiologist is essential for an effective IONM team (9). The IONM program must be discussed in detail with the anesthesiologist before it is initiated. Special considerations in TOET are whether an oro-tracheal or nasal-tracheal EMG tube should be selected and whether a muscle relaxant should be used during IONM. The discussions should be scheduled well in advance so that the anesthesiologist has sufficient time to make necessary preparations.
According to current INMSG guidelines for using IONM (21), full muscular activity should be restored as soon as possible after intubation. Spontaneous respiration and normal muscle twitch activity can usually be restored within several minutes after intubation by administration of succinyl-choline at 2–2.5 mg/kg or a small dose of a non-depolarizing muscle relaxant (e.g., rocuronium and atracurium) at 0.5 mg/kg. Lu et al. (22) used a porcine model to investigate and compare laryngeal muscle recovery profiles obtained by a standard dose (1 mg/kg) of succinylcholine, a standard dose (0.6 mg/kg) of rocuronium, and a low dose (0.3 mg/kg) of rocuronium during IONM of the RLN. To achieve an 80% recovery of the control response, the standard dose of succinylcholine required 19.7±1.5 minutes; the standard dose of rocuronium required 29.3±5.7 minutes; and the low dose of rocuronium required 16.3±2.5 minutes. The EMG signal recovery returned to baseline within 30 minutes in the standard-dose succinylcholine and in the low-dose rocuronium groups, but it did not return to baseline until 1 hour after the muscle relaxant given in the standard-dose (0.6 mg/kg) rocuronium group.
During TOET, the preparation time after intubation is almost always longer than 30 minutes before the first-time nerve stimulation; therefore a low dose or standard dose of rocuronium should be sufficient for inducing general anesthesia. However, further animal and clinical studies are needed to establish standards for the use of muscle relaxants in IONM for TOET.
In conclusion, effective use of IONM in TOET requires sufficient experience and training. Surgeons and anesthesiologists in the surgical team require adequate experience in IONM standards, equipment, trouble-shooting algorithms, limitations and failures. Nerve monitoring experience is strongly recommended before IONM is used in TOET.
Pre- and post-operative care
Obtaining written informed consent from the patient before performing TOET is mandatory and should include the following:
- Type of surgery;
- Objectives of surgery;
- Consequences of thyroidectomy;
- Risks and benefits of declining thyroidectomy;
- Risks of transoral thyroidectomy;
- Consequences of IONM, e.g., staged thyroidectomy (2.5%) and technical failure (1%).
Additionally, laryngoscopy should be routinely performed pre-operatively (L1) and post-operatively (L2) in all patients undergoing TOET. Performing these procedures can improve accuracy in predicting and evaluating improvement in glottic function after TOET.
Equipment and setup
The basic IONM equipment are the stimulating electrodes, recording electrodes, and nerve monitoring systems.
A. Stimulating electrodes
For IONM in conventional open thyroidectomy, the laryngeal EMG response is evoked by using a handheld monopolar or bipolar stimulation probe (9) or dissecting stimulator (23) to depolarize the VN or RLN. Various commercially available probes and stimulators can be used in IONM according to the stimulation requirements, the specific surgical monitoring application, and the preferences of the surgeon (24).
A major reason for the limited use of IONM in endoscopic and robotic thyroidectomies is the difficulty introducing the stimulating probe in the neck endoscopic operating space from a remote incision wound and then performing a standard IONM procedure (V1, R1, R2, V2). Four solutions have been proposed in the literature.
First, the stimulation probe can be introduced into the operating space through a percutaneous puncture on the side of the main lesion. In a prospective study of a series of thyroidectomies performed in 132 consecutive patients with 156 RLNs at risk, Zhang et al. (25) used standard IONM to perform total endoscopic thyroidectomy via bilateral breast approach. In all patients, IONM was successfully performed via percutaneous probe stimulation with no morbidity or scarring in the neck. Percutaneous probe stimulation can be performed with a standard commercially available stimulation probe. Stimulation does not require a change from the endoscopic instrument to the stimulator in the trocar and does not require additional operating space in the trocar.
Second, a specially designed stimulation probe with an increased length can be inserted from the trocar of the chest, the axilla, or the areola incision into the neck operating space (26). This stimulation probe is usually inserted through the right 5 mm port.
Third, an endoscopic grasper can be used to insert a flexible wire probe into the neck operating space(27).
Finally, a stimulating probe can be configured as a dissecting instrument or energy-based device. For example, a stimulation electrode can be connected to an endoscopic monopolar electrocauterization hook so that a single endoscopic instrument can switch between a cauterization mode and a stimulation mode. Table 1 summarizes the characteristics of different intermittent stimulation methods and their advantages and disadvantages.
Table 1
| Intermittent stimulation device | Advantages | Disadvantages |
|---|---|---|
| Percutaneous probe | V1, R1, S1, S2, R2, V2; same instrument used in open procedure; low cost; incremental probe enables remote console control and event capture; highly flexible; ball-tip probe is available; periodic continuous stimulation (V, R); does not require ports or a change in instruments | Additional step/procedure; additional neck skin incision; (e.g., two skin incisions in bilateral procedure); possible loss of CO2 insufflation; higher stimulation intensity (2–3 mA) compared to other probes; rapid tip wear; holding instruments in place can be interfered; probe cannot be reused |
| Long probe | V1, R1, S1, S2, R2, V2; no additional neck skin incision is required; low intensity stimulation (percutaneous); versatile (bilateral use); ball-tip probe is available | Dedicated long probe; instrument change; additional cost/limited availability; inflexible tip; incremental probe/remote control unavailable; some loss of CO2 insufflation from port; need for port; probe cannot be reused |
| Flexible wire probe | V1, R1, S1, S2, R2, V2; inserted into port without additional neck skin incision; low intensity stimulation; versatile (bilateral use); applicable for endoscopic and robotic surgery | Hindrance, some instrument interference; ball-tip probe unavailable; probe cannot be reused; requires additional operating space in trocar; may be displaced during use of ports |
| Endoscopic instrument | V1, R1, S1, S2, R2, V2; no additional neck skin incision; applicable for endoscopic and robotic use; dissecting and stimulation can be performed simultaneously; instrument interference is minimal; no loss of CO2 insufflation; versatile (bilateral use); same stimulation intensity compared to open surgery; can be used at high EMG amplitudes; reusable; ergonomic | Not commercially available; requires installation; requires shift between coagulation and stimulation; requires special adapter and cable |
| Energy-based device | V1, R1, S1, S2, R2, V2; enables simultaneous dissection, hemostasis, and stimulation; no additional neck skin incision; applicable for endoscopic and robotic use; versatile (bilateral use); ergonomic | Not commercially available; requires cooling time before use; cannot be reused |
Chen et al. (20) reported their preliminary experience in wire delta stimulating electrode placements for continuous VN stimulation during TOETVA. The authors noted that using these electrodes for continuous IONM in TOETVA has several potential drawbacks, which will have a longer setup time, a higher cost, a higher risk of injury to the carotid sheath organs (internal jugular vein, carotid artery, and VN), and a higher risk of cardiopulmonary effects caused by VN stimulation. Therefore, the safety and cost-effectiveness of using continuous stimulating electrodes for IONM need further study.
B. Recording electrodes
Currently, the most commonly used electrodes in commercially available monitoring systems are endotracheal tube electrodes (9). Endotracheal tube electrodes are widely available, safe, non-invasive, easy to set up, easy to use, and derive larger areas of evoked muscle potentials. The recording electrode sites have also been reported include laryngeal palpation, glottic observation, and glottic pressure monitoring, endoscopically placed intramuscular vocal cord electrodes, intramuscular electrodes placed through the cricothyroid membrane, postcricoid surface electrodes, and sub-perichondrium thyroid cartilage electrodes (9,28-32). However, none of these methods is routinely used, and none has been used in TOET.
The EMG tube used for TOET can be intubated orally or nasally. Orotracheal intubation is more common because it is faster, easier, and less susceptible to displacement caused by head movement. Orotracheal tubes are available in a wider range of sizes compared to nasal tubes and are less likely to cause contamination of the trachea. Orotracheal tubes also provide better tube/electrode contact with the vocal cords. However, disadvantages of orotracheal intubation include the greater difficulty securing the EMG tube, the risk of accidental extubation, and the hindrance of the EMG tube in the operating space. Therefore, some surgeons prefer nasotracheal intubation because it provides more operating space in TOET, is easier to secure, and has a lower extubation rate. Disadvantages of nasotracheal intubation include the greater difficulty of insertion, the greater displacement with head movement, and the potential for causing nasal problems such as laceration or trauma to the nostrils, nasal septum and turbinates. Another disadvantage is the limited availability of different EMG tube lengths and sizes needed to maximize tube/electrodes contact with the vocal cords. The contraindications for nasotracheal intubation are listed in Table 2.
Table 2
| Absolute |
| Suspected epiglottitis |
| Midfacial instability |
| Coagulopathy |
| Suspected basilar skull fracture |
| Relative |
| Large nasal polyps |
| Suspected nasal foreign bodies |
| Recent nasal surgery |
| Upper neck hematoma or infection |
| History of frequent episodes of epistaxis |
EMG, electromyography; TOET, transoral endoscopic thyroidectomy.
Malpositioned endotracheal surface electrodes can cause an IONM dysfunction and an increased risk of RLN injury. Lu et al. (33) analyzed EMG orotracheal tube placements in 105 adult patients undergoing elective thyroidectomy. Direct laryngoscopy confirmed that each EMG orotracheal tube was placed with the middle of the exposed electrodes in good contact with the true vocal cords. The IONM was successfully performed in the initial endotracheal tube position in 94.3% (n=99) of the patients. The remaining 5.7% (n=6) of the patients required further tube depth adjustment under fiberoptic bronchoscopy. Notably, the optimal mean depth significantly (P<0.01) differed between men (20.6±0.97 cm) and women (19.6±1.0 cm). The height of the subjects was significantly associated with tube depth (P<0.05).
Additionally, the EMG tube electrode position can be severely displaced when the patient is repositioned. Whenever the patient is repositioned, therefore, laryngoscopy should be repeated for the most accurate assessment of tube position. However, a separate procedure is not required (34). Although no studies have reported the prevalence of oral- or nasotracheal EMG tube displacement during TOET, the prevalence is expected to exceed that in conventional open thyroidectomy due to the increased surgical manipulation near the mouth and nose area. Therefore, the setup procedure for functional IONM during TOET should include routine verification of proper electrode position whenever laryngoscopy is repeated. Additionally, the tube insertion depth and its rotation relative to the VC should be carefully noted and monitored by both the surgeon and anesthesiologist during surgery
C. Nerve monitoring systems
Figure 1 shows the recommended operating room setup for performing IONM during TOET. The nerve monitoring systems should be positioned near the HD monitor to facilitate observation by the surgeon. Alternatives for displaying EMG responses include integration in the HD monitor, use of a console design with a mobile arm, and the use of a mini LCD screen, iPad, or Google glass.
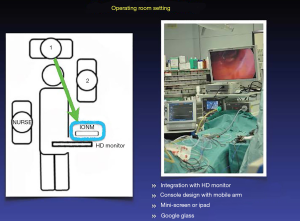
Barczynski M et al. (35), reported that IONM had a higher positive predictive value (PPV) in systems based on EMG waveform analysis (90.9%) compared to simple nerve stimulators with acoustic signaling (66.7%). Therefore, using IONM with both audio and graphic monitors has many benefits, including recording of amplitude and latency profiles, synchronization of surgical maneuvers, documentation, quantification, storage, differentiation between signals and artifacts, forensics, research, justification for surgical decision making, and review of surgical results. An LOS may be difficult to determine precisely if only audio data are monitored (9).
Standard procedures
RLN monitoring
The standard procedure for IONM in TOET is performed in four steps (V1-R1-R2-V2) (36,37).
V1 step
Before lateral thyroid dissection, an initial EMG signal is obtained by applying VN stimulation. This step is essential because it confirms a functional neuromonitoring system, provides original reference data, and detects occurrence of a non-RLN signal in a timely manner. Despite the limited operating space during TOET, VN stimulation is easily applied by performing ball-tip probe mapping on the carotid sheath with a slightly increased stimulation current (i.e., 3 mA) (25,38).
R1 step
The signal obtained from the RLN is mapped or identified at the tracheoesophageal groove. The R1 signal provides a reference datum after nerve dissection is completed.
R2 step
The signal is obtained by stimulating the most proximally exposed portion of the RLN after dissection is completed.
V2 step
The final test of the VN is performed after hemostasis of the surgical field is complete.
After the four-step IONM procedure, three signal events can be used for outcome evaluation (36,37):
(I) Stable signal
Improved or unchanged amplitude of R2 and V2 signals as compared with R1 and V1 signals confirms that the RLN and VN have not been injured during surgical dissection.
(II) LOS (defined as EMG amplitude response <100 µV)
An R2 LOS or V2 LOS after surgical dissection of the RLN may indicate RLN injury. In this case, the site of disrupted nerve conduction must be determined, and the injury mechanism must be elucidated. In a Type I injury (i.e., segmental or localized RLN injury), the site of disrupted nerve conduction can be located and classified (i.e., Type 1 segmental or localized RLN injury). Absence of disrupted nerve conduction should raise the suspicion of type II injury (i.e., diffuse or global RLN injury). Contralateral VN stimulation is then required to exclude false LOS caused by monitoring equipment dysfunction, EMG tube malposition, or misused muscle relaxants (9,39-41).
(III) Weak or incomplete LOS
Partial RLN injury caused by surgical traction, compression, clamping, mechanical trauma or electro-cauterization is characterized by a weak point of nerve conduction on the exposed RLN (i.e., amplitude reduction exceeding 100 µV in proximal RLN stimulation in comparison with distal RLN stimulation) with visual confirmation of anatomical integrity (16,36,42).
Monitoring the external branch of the superior laryngeal nerve (EBSLN)
Since IONM can be used to identify the EBSLN and to assess its functional integrity, IONM can improve voice recovery after thyroidectomy (43).
To detect intraoperative EBSLN injury, Dionigi et al. (44) added two steps to the standard procedure for using IONM in TOET: S1 for early identification of the EBSLN and for initial EBSLN stimulation followed by S2 for final EBSLN stimulation when the surgical procedure is completed, i.e., when hemostasis is achieved and the STA is ligated. That is, the proposed six-step procedure is V1-R1-S1-S2-R2-V2.
Future directions
Improved techniques and new surgical devices have facilitated the use of IONM to improve TOET outcomes. However, further studies are needed to determine whether IONM significantly improves specific IONM outcomes. For example, future studies can evaluate its effectiveness in terms of nerve palsy rate and can investigate its use for identification (neural mapping), aid in dissection, prognostication of postoperative neural function and lesion site identification. The future studies can also provide guidance for training and learning curve, serve and facilitate exploration of both thyroid lobes, and provide support for investigating new indications.
As in conventional IONM-assisted thyroidectomy, guidelines and standard procedures are needed for using IONM in TOET. Another important issue is the development of EMG tubes, dedicated probe stimulators, and other equipment specifically designed for use in TOET. Further studies are needed to compare the advantages and disadvantages of orotracheal and nasotracheal intubation approaches. The step-by-step troubleshooting algorithm assessment included in the INMSG guidelines (9) also require modification for TOET. For example, the troubleshooting procedure should include management of EMG tube dislocation, contralateral VN stimulation in case of LOS, laryngeal twitch assessment, and repeat laryngoscopy. The many potential areas of further research indicate the strong potential for further development and applications of IONM and for further technological breakthroughs that will improve the safety of TOET. The procedure can make the surgeon feel more comfortable identifying and protecting the nerve during the dissection technique, and make a novice surgeon have a sharper learning curve and reduce the likelihood of nerve injury during this period.
Acknowledgments
Funding: This study was supported by a grant from Kaohsiung Medical University Hospital (KMUH105-5R39).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Anuwong Angoon, Hoon Yub Kim, Ralph P. Tufano and Gianlorenzo Dionigi) for the series “Transoral Thyroidectomy” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.03.01). The series “Transoral Thyroidectomy” was commissioned by the editorial office without any funding or sponsorship. Gianlorenzo Dionigi served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Thyroid from Mar. 2017 to Feb. 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pai VM, Muthukumar P, Prathap A, et al. Transoral endoscopic thyroidectomy: A case report. Int J Surg Case Rep 2015;12:99-101. [Crossref] [PubMed]
- Anuwong A. Transoral Endoscopic Thyroidectomy Vestibular Approach: A Series of the First 60 Human Cases. World J Surg 2016;40:491-7. [Crossref] [PubMed]
- Anuwong A, Kim HY, Dionigi G. Transoral endoscopic thyroidectomy using vestibular approach: updates and evidences. Gland Surg 2017;6:277-84. [Crossref] [PubMed]
- Dionigi G, Lavazza M, Wu CW, et al. Transoral thyroidectomy: why is it needed? Gland Surg 2017;6:272-6. [Crossref] [PubMed]
- Richmon JD, Holsinger FC, Kandil E, et al. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg 2011;5:279-82. [Crossref] [PubMed]
- Wang Y, Yu X, Wang P, et al. Implementation of Intraoperative Neuromonitoring for Transoral Endoscopic Thyroid Surgery: A Preliminary Report. J Laparoendosc Adv Surg Tech A 2016;26:965-71. [Crossref] [PubMed]
- Jeannon JP, Orabi AA, Bruch GA, et al. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009;63:624-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Terris DJ, Snyder S, Carneiro-Pla D, et al. American Thyroid Association statement on outpatient thyroidectomy. Thyroid 2013;23:1193-202. [Crossref] [PubMed]
- Chen AY, Bernet VJ, Carty SE, et al. American Thyroid Association statement on optimal surgical management of goiter. Thyroid 2014;24:181-9. [Crossref] [PubMed]
- Musholt TJ, Clerici T, Dralle H, et al. German Association of Endocrine Surgeons practice guidelines for the surgical treatment of benign thyroid disease. Langenbecks Arch Surg 2011;396:639-49. [Crossref] [PubMed]
- Shindo ML, Caruana SM, Kandil E, et al. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck 2014;36:1379-90. [PubMed]
- Lombardi CP, Raffaelli M, Princi P, et al. Safety of video-assisted thyroidectomy versus conventional surgery. Head Neck 2005;27:58-64. [Crossref] [PubMed]
- Terris DJ, Anderson SK, Watts TL, et al. Laryngeal nerve monitoring and minimally invasive thyroid surgery: complementary technologies. Arch Otolaryngol Head Neck Surg 2007;133:1254-7. [Crossref] [PubMed]
- Dionigi G, Alesina PF, Barczynski M, et al. Recurrent laryngeal nerve injury in video-assisted thyroidectomy: lessons learned from neuromonitoring. Surg Endosc 2012;26:2601-8. [Crossref] [PubMed]
- Dionigi G, Kim HY, Wu CW, et al. Neuromonitoring in endoscopic and robotic thyroidectomy. Updates Surg 2017;69:171-9. [Crossref] [PubMed]
- Witzel K, Benhidjeb T. Monitoring of the recurrent laryngeal nerve in totally endoscopic thyroid surgery. Eur Surg Res 2009;43:72-6. [Crossref] [PubMed]
- Inabnet WB 3rd, Suh H, Fernandez-Ranvier G. Fernandez-Ranvier, Transoral endoscopic thyroidectomy vestibular approach with intraoperative nerve monitoring. Surg Endosc 2017;31:3030. [Crossref] [PubMed]
- Chen HK, Chen CL, Wen KS, et al. Application of transoral continuous intraoperative neuromonitoring in natural orifice transluminal endoscopic surgery for thyroid disease: a preliminary study. Surg Endosc 2018;32:517-25. [Crossref] [PubMed]
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Lu IC, Chang PY, Hsu HT, et al. A comparison between succinylcholine and rocuronium on the recovery profile of the laryngeal muscles during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Kaohsiung J Med Sci 2013;29:484-7. [Crossref] [PubMed]
- Chiang FY, Lu IC, Chang PY, et al. Stimulating dissecting instruments during neuromonitoring of RLN in thyroid surgery. Laryngoscope 2015;125:2832-7. [Crossref] [PubMed]
- Wu CW, Liu X, Barczyński M, et al. Optimal stimulation during monitored thyroid surgery: EMG response characteristics in a porcine model. Laryngoscope 2017;127:998-1005. [Crossref] [PubMed]
- Zhang D, Li F, Wu CW, et al. Percutaneous probe stimulation for intraoperative neuromonitoring in total endoscopic thyroidectomy: A preliminary experience. Head Neck 2017;39:1001-7. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Neuromonitoring and video-assisted thyroidectomy: a prospective, randomized case-control evaluation. Surg Endosc 2009;23:996-1003. [Crossref] [PubMed]
- Dionigi G, Chiang FY, Hui S, et al. Continuous Intraoperative Neuromonitoring (C-IONM) Technique with the Automatic Periodic Stimulating (APS) Accessory for Conventional and Endoscopic Thyroid Surgery. Surg Technol Int 2015;26:101-14. [PubMed]
- Alon EE, Hinni ML. Transcricothyroid electromyographic monitoring of the recurrent laryngeal nerve. Laryngoscope 2009;119:1918-21. [Crossref] [PubMed]
- Petro ML, Schweinfurth JM, Petro AB. Transcricothyroid, intraoperative monitoring of the vagus nerve. Arch Otolaryngol Head Neck Surg 2006;132:624-8. [Crossref] [PubMed]
- Randolph GW, Kobler JB, Wilkins J. Recurrent laryngeal nerve identification and assessment during thyroid surgery: laryngeal palpation. World J Surg 2004;28:755-60. [Crossref] [PubMed]
- Liddy W, Barber SR, Lin BM, et al. Monitoring of the posterior cricoarytenoid muscle represents another option for neural monitoring during thyroid surgery: Normative vagal and recurrent laryngeal nerve posterior cricoarytenoid muscle electromyographic data. Laryngoscope 2018;128:283-9. [Crossref] [PubMed]
- Chiang FY, Lu IC, Chang PY, et al. Comparison of EMG signals recorded by surface electrodes on endotracheal tube and thyroid cartilage during monitored thyroidectomy. Kaohsiung J Med Sci 2017;33:503-9. [Crossref] [PubMed]
- Lu IC, Chu KS, Tsai CJ, et al. Optimal depth of NIM EMG endotracheal tube for intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroidectomy. World J Surg 2008;32:1935-9. [Crossref] [PubMed]
- Tsai CJ, Tseng KY, Wang FY, et al. Electromyographic endotracheal tube placement during thyroid surgery in neuromonitoring of recurrent laryngeal nerve. Kaohsiung J Med Sci 2011;27:96-101. [Crossref] [PubMed]
- Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg 2014;38:599-606. [Crossref] [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [Crossref] [PubMed]
- Chiang FY, Lee KW, Chen HC, et al. Standardization of Intraoperative Neuromonitoring of Recurrent Laryngeal Nerve in Thyroid Operation. World J Surg 2010;34:223-9. [Crossref] [PubMed]
- Wu CW, Dionigi G, Chen HC, et al. Vagal nerve stimulation without dissecting the carotid sheath during intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Head Neck 2013;35:1443-7. [PubMed]
- Randolph GW, Dionigi G, Chen HC, et al. The Recurrent and Superior Laryngeal Nerves. Cham (ZG): Springer International Publishing, 2016.
- Randolph GW. Surgery of the Thyroid and Parathyroid Glands. Philadelphia, PA: Saunders, Elsevier, 2013.
- Wu CW, Wang MH, Chen CC, et al. Loss of signal in recurrent nerve neuromonitoring: causes and management. Gland Surg 2015;4:19-26. [PubMed]
- Snyder SK, Lairmore TC, Hendricks JC, et al. Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg 2008;206:123-30. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123:S1-14. [Crossref] [PubMed]
- Dionigi G, Kim HY, Randolph GW, et al. Prospective validation study of Cernea classification for predicting EMG alterations of the external branch of the superior laryngeal nerve. Surg Today 2016;46:785-91. [Crossref] [PubMed]
Cite this article as: Huang TY, Catalfamo A, Wu CW, Chiang FY, Dionigi G. Neural monitoring in transoral endoscopic thyroidectomy. Ann Thyroid 2018;3:7.


