
Active surveillance for very low-risk papillary thyroid carcinoma: experience and perspectives from Japan
The annual incidence of thyroid cancer has recently been around 14,000 cases in Japan and more than 50,000 in the United States. In countries with sufficient iodine intake, papillary thyroid carcinoma (PTC) accounts for over 90% of all thyroid cancers. The latest keywords in the management of PTC are: (I) risk-adapted management; and (II) countermeasures against overdiagnosis and overtreatment.
Risk-adapted management of PTC based on a risk-group classification system
The majority of patients with PTC are low-risk and show excellent prognosis, but conventional policies on the treatment of PTC have differed between countries. In Western countries, patients have usually been treated with total thyroidectomy and radioactive iodine (RAI) remnant ablation followed by life-long thyroid-stimulating hormone (TSH) suppression therapy and periodic measurement of thyroglobulin concentrations. On the other hand, patients are usually treated with thyroid-conserving surgery (lobectomy) and followed-up using ultrasonography (US) without postoperative adjuvant therapies in Japan.
At the Division of Head and Neck at Cancer Institute Hospital (CIH), a tertiary oncology referral center in Tokyo, Japan, we retrospectively analyzed a total of 604 patients who had undergone initial surgery for PTC >1 cm in diameter between 1976 and 1998. Mean duration of follow-up was 11 years. Multivariate analysis for cause-specific survival (CSS) identified distant metastasis as the only significant risk factor for younger patients (<50 years). For older patients (≥50 years), distant metastasis, massive extrathyroidal invasion (with preoperative recurrent laryngeal nerve palsy, transluminal invasion of the trachea/esophagus), and large nodal metastasis (≥3 cm) were identified as significant factors. From these results, younger patients with distant metastasis and older patients with any of the three factors were defined as high-risk, while all other patients were defined as low-risk. Overall, 106 high-risk patients (18%) and 498 low-risk patients (82%) displayed 10-year CSS rates of 69% and 99%, respectively (1). From 2005 onward, we adopted our own risk-adapted approach to patients with PTC based on the risk-group classification system. Patients classified into the high-risk group receive total thyroidectomy with adjuvant therapies, while the extent of thyroidectomy in patients with unilateral low-risk PTC is determined based on the choices of the patient. We recently verified the validity of our risk-group definition and evaluated treatment outcomes for low-risk patients according to the extent of surgery. Of the 1,187 patients who underwent initial surgery for PTC (diameter >1 cm) between 1993 and 2010, a total of 967 (81%) were classified as low-risk and showed a 10-year CSS of 99% (Figure 1). Among these low-risk patients, 791 (82%) underwent less-than-total thyroidectomy. Ten-year CSS and disease-free survival (DFS) rates did not differ between patients who received total thyroidectomy and those who underwent less-than-total thyroidectomy (Table 1) (2).
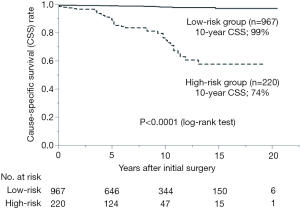
Table 1
| Characteristic | Less than total | Total or near-total | P (log-rank test) |
|---|---|---|---|
| No. of patients | 791 (82%) | 176 (18%) | |
| Recurrence | 67 (9%) | 12 (7%) | |
| 10-year DFS | 87% | 91% | 0.90 |
| Location of recurrence | |||
| Lymph node | 52 (7%) | 11 (6%) | |
| Remnant thyroid | 4 (0.5%) | 0 | |
| Other neck | 6 (0.8%) | 1 (0.6%) | |
| Distant | 32 (4%) | 5 (3%) | |
| Cause-specific death | 9 (1%) | 2 (1%) | |
| 10-year CSS | 99% | 99% | 0.61 |
DFS, disease-free survival; CSS, cause-specific survival.
In 2010, the Japanese Clinical Guidelines (3) adopted risk-adapted management for patients with PTC, and lobectomy was recommended for low-risk T1N0M0 patients. High-risk PTC was defined as a tumor >5 cm in diameter, extrathyroidal extension to the mucosa of the trachea/esophagus, lymph node metastasis >3 cm, or extension into surrounding organs and distant metastasis at diagnosis. Total thyroidectomy was recommended for patients displaying one or more of these characteristics. Other patients were classified as falling within a “gray zone” (intermediate-risk group) and the guidelines stated that the extent of surgery should be individually determined based on the balance between incidences of surgical complications and prediction of CSS and DFS evaluated using the risk classification system of the individual institution at which the patient is undergoing surgery.
According to the 2009 revision of the American Thyroid Association (ATA) guidelines (4), the initial surgical procedure should be near-total or total thyroidectomy for patients with thyroid cancer >1 cm in diameter. However, given the accumulated evidence showing excellent survival for patients with low-risk PTC regardless of the extent of thyroidectomy and the lower risk of surgical complications from lobectomy (5-10), the 2015 revision of the ATA guidelines (11) introduced the concept of risk-adapted management and remarked that lobectomy alone may provide sufficient initial treatment for low-risk PTC. In that edition of the guidelines, high-risk PTC was defined as that with tumor diameter >4 cm, gross extrathyroidal extension, or clinically apparent metastatic disease to nodal or distant sites. For those patients, the guidelines stated that the initial surgical procedure should include near-total or total thyroidectomy. On the other hand, for patients with PTC <1 cm without extrathyroidal extension and clinical metastasis, the procedure should be thyroid lobectomy. For patients with PTC 1–4 cm in diameter without extrathyroidal extension or metastasis, either a bilateral or unilateral procedure can be selected. After years of debate, treatment policies for PTC in the East and West have increasingly become integrated under the concept of risk-adapted management.
Overdiagnosis and overtreatment of thyroid cancers
Over the last few decades, the incidence of thyroid cancer has continued to increase all over the world. In the United States, Davies et al. (12) reported that the incidence of thyroid cancer increased from 3.6 per 100,000 in 1973 to 8.7 per 100,000 in 2002. This has mainly been attributed to the increased detection of small PTCs with the increasingly greater sensitivity of diagnostic procedures such as US, rather than from true increases in occurrence rates. Indeed, increased detection and surgery for these carcinomas has not resulted in decreases in the mortality rates from thyroid cancer. Debate on the management of these subclinical thyroid cancers is therefore growing.
Discrepancies are well-known to exist between clinical and subclinical PTCs. The clinical incidence of PTC is known to be only about 0.1–0.05% in the general population, but many autopsy series from various countries have shown a surprisingly high prevalence of latent PTC, ranging from 8.6% to 35.6% among patients dying from diseases other than thyroid cancer (13-17). Those studies have also shown that the prevalence of such tumors increases steeply from birth to adulthood and remains relatively constant thereafter. According to a recent meta-analysis of 35 studies [1949–2007] covering 12,834 autopsies (18), the prevalence of differentiated thyroid cancer (DTC) among whole gland examination was 11.2% [95% confidence interval (CI), 6.7–16.1%]. This prevalence stabilized from 1970 onward, and no time effect was observed. The authors of the meta-analysis stated that current increasing incidence of DTC is unlikely to reflect a true increase in tumorigenesis. Moreover, the prevalence of PTC in health screenings using a recently developed high-end US was reported as 3.5% (19). Those minute PTCs are usually thought to remain innocent and asymptomatic throughout the life of the patient.
Advances in the accuracy of health check devices and increasing opportunities for cancer screening among healthy subjects have accelerated screening effects, and individuals diagnosed with thyroid cancer usually end up undergoing surgical treatment. The incidence of thyroid cancer has not stopped increasing in the United States (20). Furthermore, in Korea, the rate of diagnosis of thyroid cancer showed a 15-fold increase from 1993 to 2011, presumably as a result of a national cancer screening program initiated by the government in 1999. Thyroid cancer has now become the most common cancer among women in Korea. Among those cancers, 56% were ≤1 cm and 25% were ≤5 mm. The mortality rate associated with thyroid cancer has remained stable. Ahn et al. called the phenomenon the “thyroid cancer epidemic” (21). Meanwhile, effective countermeasures to minimize the overdiagnosis and overtreatment of thyroid cancer have increasingly been explored.
Countermeasures for overdiagnosis and overtreatment of thyroid cancer
Three main measures are used to prevent the overdiagnosis and overtreatment of PTC.
(I) A new standard for cancer screening and indications for fine needle aspiration (FNA)
The first is to establish a new standard for cancer screening and clinical diagnosis. In Japan, we have reached the consensus that detecting papillary microcarcinoma (PMC) in mass screening offers no advantages to the general population. The Japanese guidebook for ultrasound diagnosis of thyroid diseases published in 2012 stated that setting examination methods and a standard for scrutiny beforehand is important to avoid harm to the examinee. As for the procedure for diagnosing solid thyroid nodule, the guidebook recommended observation without FNA for nodules ≤5 mm (22). Thanks to such considerations, the increase in the rate of thyroid cancer incidence in Japan has not been particularly marked compared to that in other countries (23). The 2015 ATA guidelines also restricted the indications for FNA for thyroid nodules, recommending that US-suspicious thyroid nodules of 1 cm or smaller without evidence of extrathyroidal extension or suspicious lymph nodes may be observed with close follow-up, rather than pursuing immediate FNA (11). Moreover, the United States Preventive Services Task Force recently published a statement that recommended against screening for thyroid cancer in asymptomatic adults (24).
(II) Changes in diagnostic criteria or nomenclature for indolent thyroid tumors
The second measure is to change the diagnostic criteria or terminology for thyroid tumors, as been proposed by thyroid pathologists. In 2003, the Porto proposal advocated renaming PMC as papillary microtumor to avoid overtreatment and psychological anxiety in patients. This terminology was recommended to be adopted for incidental tumors detected on pathological examination of thyroid glands resected due to other thyroid diseases or for tumors discovered incidentally on imaging studies such as US, computed tomography (CT) or magnetic resonance imaging (MRI). Patients younger than 20 years and tumors with extrathyroidal invasion, vascular invasion and/or poorly differentiated component were excluded, but those with multiple PMCs were not (25). Revision of the nomenclature for encapsulated follicular variant of PTC (EFVPTC) has also been suggested (26). Nikiforov et al. proposed reclassification of noninvasive EFVPTC (malignant disease) as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP, borderline disease) and claimed that the revision would affect a large population of patients and result in a significant reduction in psychological and clinical consequences associated with the diagnosis of “cancer”. The 4th edition of the World Health Organization (WHO) Classification of Tumours of Endocrine Organs published in 2017 adopted the concept of NIFTP as one of the borderline tumors showing extremely low malignant potential (27).
(III) Active surveillance trials for asymptomatic PMC
The last resort to stop overtreatment for patients who have already been diagnosed with thyroid cancer is active surveillance for selected low-risk PMC. Since the 1990s, two Japanese institutions (Kuma Hospital in Kobe, a hospital specializing in thyroid diseases, and CIH) have initiated prospective clinical trials of active surveillance for asymptomatic PMC.
Prospective trials of active surveillance for asymptomatic PMC in Japan
At CIH, we retrospectively reviewed outcomes for 178 patients who underwent surgery for PMC defined as PTC with a maximum diameter of 1.0 cm between 1976 and 1993 to determine prognostic factors. The most significant risk factors were the presence of clinically apparent lymph node metastasis and hoarseness due to recurrent laryngeal nerve palsy at the time of diagnosis. All four cases of distant metastasis and four cases of cancer-specific death occurred in 30 patients with symptomatic PMC who showed either cervical lymphadenopathy >1 cm in diameter or recurrent nerve invasion, or both. On the other hand, neither distant metastasis nor cause-specific death was seen in the remaining 148 patients without those symptoms (28).
Following these results, we started a prospective clinical trial of active surveillance for asymptomatic PMC (cT1aN0M0) in 1995 (29). Patients with PMC diagnosed by US-guided FNA cytology were evaluated for the presence of distant metastasis, clinically apparent lymphadenopathy (>1 cm), or extrathyroidal invasion using modalities such as neck US, chest CT and laryngoscopy. For patients with asymptomatic PMC, we provided information regarding the option of immediate surgery and active surveillance. After that, treatment choice was determined based on the informed decisions of the patient. In cases of active surveillance, the tumor was surveyed by modalities such as US every 6 or 12 months. We recommend surgery during follow-up if the patient meets the following criteria: (I) change in patient preference to surgery; (II) the tumor has grown beyond the posterior wall of the thyroid, approaching the recurrent laryngeal nerve and/or trachea; (III) development of clinically evident lymph node metastasis or distant metastasis; or (IV) increase in tumor size to over 1 cm.
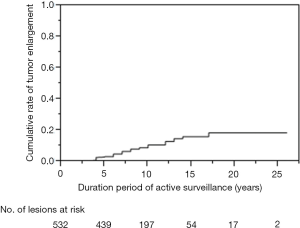
As of 2013, active surveillance has been chosen over immediate surgery by 426 of 475 patients (90%) with asymptomatic PMC. These patients comprised 55 men and 371 women, with a mean age of 54 years (range, 23–84 years). In the majority of patients (n=353; 83%), PTC was incidentally detected by US, followed by fluorodeoxyglucose positron emission tomography (n=27), CT/MRI (n=25), palpation (n=11) and microscopic lymph node metastasis detected from neck dissection for other head and neck malignancies (n=10). Because CIH is an oncology center, 108 patients (25%) also suffered from other kinds of malignancy. Eighty-one patients (19%) had multiple PMCs. In total, 532 PMC lesions had been followed. Increased or decreased tumor size on US was defined as a change in maximum tumor diameter ≥3 mm, from the beginning of observation. After a mean follow-up of 8.7 years (range, 1–26 years), 45 of 532 lesions (8%) had increased in size. However, 455 lesions (86%) had shown no change and 32 lesions (6%) had decreased in size. The cumulative rate of tumor enlargement was shown in Figure 2. This rate was 3.0% at 5 years, 10.6% at 10 years and 18.4% at 20 years of follow-up. Four patients (0.9%) developed clinically evident lymph node metastasis after 4–5 years of follow-up; three in the lateral neck and one in the central neck. Eventually, 44 patients (10%) underwent surgery at 1–16 years (mean ± SD, 5.2±3.6 years) after the initial choice of active surveillance. The extent of thyroidectomy was total/near-total for 13 patients and less-than-total for 31 patients. Lateral neck dissection was only performed for three patients who developed clinical lymph node metastasis in the region. The reasons for operation were as follows: tumor enlargement (n=23; 5%), development of lymph node metastasis (n=4; 0.9%), progression to the posterior wall of the thyroid (n=1; no extrathyroidal invasion identified at the surgery), emergence of another PTC >1 cm (n=1), and primary hyperparathyroidism (n=1). The other 14 patients (3%) underwent surgery regardless of unchanged tumor status. Postoperative courses were uniformly favorable and no surgical complications, cancer recurrence or cause-specific deaths occurred. None of these patients have developed extrathyroidal extension or distant metastasis during follow-up.
A similar prospective trial has been underway at Kuma Hospital since 1993 (30). That research group recently reported the results of active surveillance performed on 1,235 patients with a mean follow-up period of 75 months (range, 10–227 months). Tumor enlargement was seen in 4.9% of patients at 5 years and in 8.0% at 10 years. Similar to our own findings, no cases showing distant metastasis or extrathyroidal invasion were encountered, although lymph node metastasis had developed in 1.7% of patients at 5 years and 3.8% at 10 years (31). These prospective trials in Japan following nearly 2,000 patients with cT1aN0M0 PTC revealed that the vast majority of tumors did not progress during active surveillance and outcomes were not markedly affected by delays in rescue surgery. As a result, the Japanese Clinical Guidelines (3) adopted the policy of active surveillance for asymptomatic PMC, representing the first such policy in the world. Those guidelines stated that surgical treatment is indicated for PMC patients with clinical lymph node metastasis on palpation or imaging studies, distant metastasis, or significant extrathyroidal extension. However, patients without these features represent candidates for observation after extensive explanation of the situation and acquisition of informed consent. The 2015 ATA guidelines (11) followed that policy and described an active surveillance management approach as an alternative to immediate surgery in patients with very low-risk tumors, such as asymptomatic PMCs.
Evidence supporting active surveillance as an alternative for patients with very low-risk PTC
Recently, experiences with active surveillance approaches to low-risk PTC outside of Japan have been reported. From the United States, investigators at Memorial Sloan Kettering Cancer Center (MSKCC) conducted active surveillance of 291 patients with intrathyroidal PTC ≤1.5 cm. During a median follow-up period of 25 months (range, 6–166 months), growth in tumor diameter of ≥3 mm was observed in 11 of 291 patients (3.8%), with a cumulative incidence of 2.5% at 2 years and 12.1% at 5 years. No regional or distant metastases developed during active surveillance (32). At Asan Medical Center, Seoul, Korea, 192 patients with PMC underwent active surveillance for >1 year, with a median follow-up of 30 months. Tumor size increased in 27 patients (14%) and 1 patient (0.5%) showed new appearance of cervical lymph node metastasis at 3 years after the initial diagnosis, but no patients fell into critical situations during observation (33).
Oda et al. (34) compared the incidence of unfavorable events between patients choosing active surveillance (n=1,179) and patients who underwent immediate surgery (n=974) using data from Kuma Hospital. In the active surveillance group, 94 patients (8.0%) underwent surgery afterwards. One of these patients (0.08%) experienced nodal recurrence, while 5 patients (0.5%) in the immediate surgery group showed recurrence at the cervical node or remnant thyroid. The immediate surgery group showed significantly higher incidences of transient vocal cord paralysis and transient and permanent hypoparathyroidism than the active-surveillance group (4.1% vs. 0.6%; 16.7% vs. 2.8%; and 1.6% vs. 0.08%, respectively; P<0.0001 each). The proportion of patients on levothyroxine supplementation was significantly larger in the immediate surgery group (66.1%) than in the active surveillance group (20.7%, P<0.0001). They concluded that the oncological outcomes were similarly excellent, but the incidences of unfavorable events were definitely higher in the immediate surgery group.
In addition, Oda et al. (35) reported on the medical costs of management for low-risk PMC, comparing active surveillance and immediate surgery. They created a model for the flow of these managements, including the steps of diagnosis, surgery, prescription of pharmacotherapies, recurrence and delayed rescue surgery for recurrence. The 10-year total cost of immediate surgery (USD 7,225) was 4.1-times more expensive than active surveillance (USD 1,525) under the Japanese healthcare insurance system. On the basis of these findings, Miyauchi et al. (36) claimed that active surveillance can be used as first-line management for low-risk PMC.
Relationship between progression of PMC and age, calcification pattern and vascularity: speculation on the natural history of low-risk PMC
Age is known as one of the most important prognostic factors for PTC, and high-risk thyroid cancers usually occur among older patients. However, the outcomes of active surveillance indicated that the reverse was the case for low-risk PMC and older patients showed less-progressive disease than younger patients. Ito et al. (31) at Kuma Hospital reported the 10-year progression rate of PMC under observation was 2.5% for patients 60 years or older, 4.9% for 40–59 years, and 22.5% for those under 40 years old.The CIH series showed a similar tendency (Table 2). Old patients with low-risk PMC might be the best candidates for observation.
Table 2
| Age at diagnosis (years) | No. of patients | No. of tumor enlargements (%) | Cumulative rate of tumor enlargement (%) | P (log-rank test) | |
|---|---|---|---|---|---|
| 5-year | 10-year | ||||
| <40 | 62 | 10 (16.1) | 7.5 | 25.4 | 0.022 for <40 vs. ≥40 |
| 40–59 | 290 | 23 (7.9) | 2.2 | 9.5 | |
| ≥60 | 180 | 13 (7.2) | 3.0 | 7.9 | |
At CIH, we have studied time-dependent changes in calcification patterns and vascularity of PMC on US during active surveillance (37). We classified ultrasound patterns of calcification as no calcification, microcalcification, macrocalcification or rim calcification (Figure 3A,B,C,D). Tumor vascularity was divided into rich or poor via color Doppler US (Figure 3E,F). The initial grade of tumor calcification correlated with age at diagnosis. PMCs in older patients showed significantly stronger calcification patterns. The incidence of tumor enlargement tended to be inversely related to the initial grade of calcification and none of the lesions displaying rim calcification showed progression (Table 3). The cumulative rate of upgrade in calcification was 25.1% at 5 years and 51.8% at 10 years (Figure 4). Lesions with initially rich vascularity exhibited a significantly higher rate of tumor enlargement (14.3%) than those with poor vascularity (4.6%, P=0.0017), but the majority (61.4%) of tumors with initially rich vascularity showed decreased blood supply during follow-up. Lesions with rich vascularity at the final examination showed a higher probability of tumor enlargement (Table 4). Time-dependent consolidation of calcification and loss of vascularity might thus be the natural course of asymptomatic PMC. Patients with PMC attaining strong calcification and poor vascularity are thought to be good candidates for active surveillance.
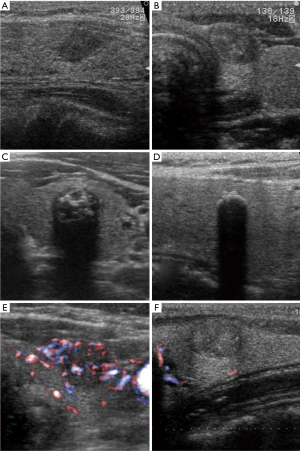
Table 3
| Characteristic | No calcification (n=135) | Microcalcification (n=235) | Macrocalcification (n=95) | Rim calcification (n=15) | P |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 52.1±11.1 | 54.2±11.9 | 56.3±11.8 | 60.1±11.5 | 0.016 |
| Change in maximum tumor diameter, n (%) | 0.14 | ||||
| Increase ≥3 mm | 13 (9.6) | 13 (5.5) | 3 (3.2) | 0 (0) | |
| No change* or decrease ≥3 mm | 122 (90.4) | 222 (94.5) | 92 (96.8) | 15 [100] |
*, no change, includes values of increase >0 but <3 mm or decrease >0 but <3 mm.
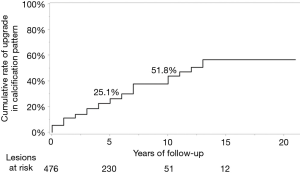
Table 4
| Characteristic | Rich# (n=70) | Poor# (n=410) | P | |||
|---|---|---|---|---|---|---|
| Poor& | Rich& | Poor& | Rich& | |||
| Number of lesions | 43 | 27 | 399 | 11 | ||
| Age at diagnosis (years) | 52.9±10.0 | 54.0±10.3 | 54.4±11.9 | 54.9±17.2 | ||
| Change in maximum tumor diameter, n (%) | <0.0001 for last vascularity rich vs. poor | |||||
| Increase ≥3 mm | 3 (7.0) | 7 (25.9) | 17 (4.3) | 2 (18.2) | ||
| No change* or decrease ≥3 mm | 40 (93.0) | 20 (74.1) | 382 (95.7) | 9 (81.8) | ||
*, no change, includes values of increase >0 but <3 mm or decrease >0 but <3 mm; #, initial vascularity; &, final vascularity.
According to these studies, we hypothesized the following natural history of thyroid cancer: (I) low-risk PTC progresses to some extent during the younger period, but might stop growing thereafter; and (II) rate of transformation from low-risk PTC to high-risk cancer might be far less than 0.1% because we no such cases have been encountered among these active surveillance trials. Active surveillance may be argued to only delay surgical intervention, and thus may represent wasted time, and younger patients should thus be treated with surgery as early as possible. Miyauchi et al. (38) recently calculated the lifetime probability of disease progression of PMC according to the initial age. Estimates were 60.3% for patients in their 20s, 37.1% in their 30s, 27.3% in their 40s, 14.9% in their 50s, 9.9% in their 60s and 3.5% in their 70s. The study indicated that 40% and 63% of patients in their 20s and 30s would not require surgical intervention during their entire life. Delaying surgical intervention can lead to patients undergoing surgery when their life circumstances improve and patients can maintain normal thyroid function for many more years.
Predictors of PMC progression other than age
In Japanese series (29,31), sex, family history of thyroid cancer, maximum diameter of tumor at diagnosis and multiplicity lesions of PMC were not significantly related to the progression of PMC during active surveillance. Reliable predictors that can anticipate progression of asymptomatic PMC to clinical disease in advance are being sought.
(I) TSH
TSH plays a key role in the initiation and progression of DTC. We investigated the association between serum TSH concentration and growth of asymptomatic PMC, but no significant association between TSH and tumor progression was verified during active surveillance (39). The study examined the course of 415 PMCs in 322 patients with 2–22 years of active surveillance (mean, 6.5 years). Both baseline TSH and mean TSH during follow-up for PMC that increased in size did not differ significantly from those of lesions that remained unchanged or decreased in size (1.79±0.25 vs. 1.80±0.06 mIU/L, P=0.47; 1.91±0.20 vs. 1.78±0.05 mIU/L, P=0.31, respectively). Increases in size were seen in 0 of 18 (0%), 15 of 260 (6%), 10 of 126 (8%), and 0 of 11 patients (0%) for PMCs with baseline TSH <0.50, 0.50–1.99, 2.00–3.99, and ≥4.0 mIU/L, respectively. A logistic regression model analyzing the association between baseline TSH and outcome showed an odds ratio of 1.01 (95% CI, 0.66–1.29). No significant correlations were apparent between mean TSH during follow-up and change in PMC volume (r=0.019, P=0.70). TSH was not a good predictor of PMC growth in CIH series. Moreover, the presence of anti-thyroglobulin/anti-thyroid peroxidase antibodies at diagnosis was unrelated to the outcomes of active surveillance for patients with PMC.
On the other hand, Kim et al. (40) demonstrated a positive association between serum TSH level and growth of PMC using a Korean series with 127 PMCs in 126 patients. During a median follow-up of 26 months, PMC progression (defined as a volume increase by ≥50% from the baseline was detected in 25 patients (19.8%). The adjusted hazard risk (HR) for PMC progression in the highest time-weighted TSH group was significantly higher (3.55; 95% CI, 1.22–10.28; P=0.02) than that in the lowest time-weighted TSH group. Elevation of serum TSH level (cut-off point: 2.50 mIU/L) was associated with PMC progression. Thus, Kim et al. (40) advocated maintaining a low-normal TSH range during active surveillance for PMC. Ito et al. (31) also showed that only 1 patient progressed among 51 patients who underwent TSH suppression therapy during active surveillance. Future prospective studies will be necessary to verify the benefits and harms of TSH suppression on PMC under observation.
(II) Molecular and pathological findings
The presence of BRAFV600E and TERT promoter mutations were reported to be associated with the prognosis of PTC. However, a preliminary report from Kuma Hospital showed that the prevalence of BRAFV600E mutation was unrelated to the outcomes of active surveillance (positive rate: 64% for 11 non-progressive PMCs, 70% for 10 enlarged PMCs, 80% for 5 PMCs that developed lymph node metastasis). No PMCs showed TERT promoter mutations (41).
Pathological findings of PMC with progression during active surveillance were also reported from Kuma Hospital (42). A high Ki-67 labeling index was associated with tumor enlargement. Intra-glandular dissemination and psammoma bodies were often seen in PMCs that developed clinical lymph node metastasis. These findings are thought to be indicators of lymph vessel infiltration of the tumor. Indeed, three of the four patients (75%) who showed lymph node metastasis during observation in the CIH series displayed multiple PMCs in the thyroid.
Practical issues in active surveillance for low-risk PTC
(I) Indications and contraindications for active surveillance
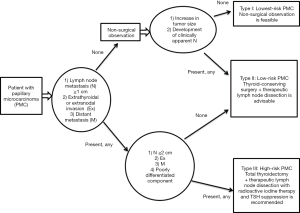
As for symptomatic PMC, we found that either extrathyroidal or extranodal invasion, or large nodal metastasis (≥2 cm) were significant risk factors for cancer-specific death. Patients with neither risk factor never died of the disease, whereas the 10-year CSS rate for patients with either risk was 74% (29). Accordingly, we identified three biologically different kinds of PMC requiring risk-adapted management. Type I comprises incidentally detected PMC without any symptoms, which is harmless and the lowest-risk cancer. Active surveillance is feasible. Type II involves the usual low-risk PTC, which can sometimes be discriminated from type I after active surveillance. This can be treated using conservative, limited thyroidectomy. Type III is symptomatic PMC with the risk factors described above, representing high-risk cancer. This is a contraindication for observation, and immediate aggressive treatment (total thyroidectomy with RAI therapy) is mandatory (Figure 5).
To predict extrathyroidal invasion of PMC, Ito et al. (43) reported that no PMCs <7 mm showed invasion to the recurrent laryngeal nerve or trachea. They proposed the risk classification of recurrent nerve invasion according to the normal rim between the tumor and posterior wall of the thyroid on preoperative imaging examinations. Significant invasion requiring partial-layer dissection of the recurrent nerve or complete dissection with reconstruction was only seen in the high-risk group without a normal rim. The risk for tracheal invasion was classified by the angle between the tumor and tracheal wall, with an acute angle representing low-risk tumor and an obtuse angle representing high-risk tumor. Significant invasions requiring tracheal cartilage resection with or without resection of the tracheal mucosa were also only seen in the high-risk group.
To facilitate safe active surveillance management in practice, the thyroid cancer management team at MSKCC collaborated with Kuma Hospital and developed a risk-stratified decision-making framework to select candidates for active surveillance. The framework consisted of three domains: (I) tumor/neck characteristics on US (size of the primary tumor, location of the tumor within the thyroid gland); (II) patient characteristics (age, comorbidities, willingness to accept observation); and (III) medical team characteristics (availability and experience of the multidisciplinary team). Based on these criteria, patients with PMC can be classified as ideal, appropriate and inappropriate candidates for active surveillance. The factors identified as showing a case is inappropriate for observation were as follows: (I) tumor/neck US characteristics with evidence of aggressive cytology on FNA, subcapsular locations adjacent to the recurrent laryngeal nerve, evidence of extrathyroidal extension, lymph node metastasis, and distant metastasis; (II) patient characteristics such as young age (<18 years), low likelihood of compliance with follow-up plans and unwillingness to accept an observational approach; and (III) medical team characteristics of unavailability of reliable neck US or little experience with thyroid cancer management (44).
For a safe and beneficial management of PMC, establishing an experienced multidisciplinary team is crucial. Certified endocrinologists or surgeons have to play a central role in educating not only health professionals, but also patients, to guarantee the quality of active surveillance.
(II) Possibility of expanding indications for active surveillance to T1b tumor
Anderson et al. (45) reported the treatment outcomes of T1 DTC using big data from the United States, including the National Cancer Data Base (T1a: 98,111 cases, T1b: 51,801 cases) and the Surveillance, Epidemiology and End Results program (T1a: 11,208 cases, T1b: 7,173 cases). After adjustment, overall (P=0.23) and disease-specific survival (P=0.93) were similar among patients with T1a versus T1b tumors. According to a study by Griffin et al. (46), increasing the size threshold for active surveillance of PTC from 1 to 1.5 cm led to a change from only 6% to 23% of patients being eligible for active surveillance based on the above-mentioned MSKCC criteria (44). Indeed, a trial from the center (32) included 59 patients with intrathyroidal T1b tumor (11–15 mm). Among those, 2 patients (3.4%) showed a tumor diameter increase of ≥3 mm during the median follow-up of 25 months. This was not significantly different from patients with T1a tumor.
We generally recommended surgery for patients with cT1bN0M0 PTC, but if patients have requested observation, the final recommendation was made by the physician taking into consideration age, tumor size, and other factors. During the period between 1995 and 2013, a total of 61 of 392 patients (16%) with cT1bN0M0 PTC underwent active surveillance. Tumor size was significantly smaller in the active surveillance group (mean, 11.7 mm; range, 11–16 mm) than in the immediate surgery group (mean, 14.5 mm; range, 11–20 mm). Among them, 4 patients (7%) showed tumor size enlargement and 2 patients (3%) developed clinically evident lymph node metastasis at a mean follow-up of 7.9 years (range, 1–17 years). No significant difference in progression rate was evident between T1a and T1b (Table 5). No postoperative recurrence was seen in patients who underwent immediate or delayed rescue surgery with tumors <15 mm in diameter. Active surveillance management would be admitted for selected T1bN0M0 PTC, but expansion of the indications for active surveillance to bigger tumors needs further careful investigation.
Table 5
| Characteristics | cT1aN0M0 Active surveillance (n=360) | cT1bN0M0 | |
|---|---|---|---|
| Active surveillance (n=61) | Immediate surgery (n=331) | ||
| Sex (male) | 41 (11%) | 14 (23%) | 52 (16%) |
| Age (years), mean ± SD (range) | 53.9±12.0 [23–84] | 54.4±10.7 [32–78] | 51.9±12.6 [17–82] |
| Tumor size (mm), mean ± SD (range) | 7.6±1.8 [2–10] | 11.7±1.1 [11–16] | 14.5±2.8 [11–20] |
| Follow-up (years), mean ± SD | 7.3±4.3 | 7.9±4.0 | – |
| Tumor size enlargement | 29 (8%) | 4 (7%) | – |
| Development of clinical lymph node metastasis | 3 (0.8%) | 2 (3%) | – |
| 5-year progression rate (%) | 5 | 5 | – |
| 10-year progression rate (%) | 12 | 12 | – |
Future tasks for active surveillance and perspectives
Several clinical issues remain unsolved regarding the management of asymptomatic PMC. For example, the difference between guidelines regarding indications for FNA for suspicious thyroid nodule would be problematic. The ATA guidelines (11) have stated that asymptomatic nodules ≤1 cm in diameter can be observed without FNA, while the Japanese guidebook (22) set the borderline at 5 mm. As for criteria for tumor size enlargement during active surveillance, Japanese clinical trials (29-31) have simply defined an increase of ≥3 mm in maximum diameter from the beginning. However, investigators at MSKCC recommended three-dimensional measurement of tumor volume, as this allowed earlier identification of growth (32). They defined a volume change >50% from baseline as meaningful. The latter definition led to a higher rate of tumor size enlargement than the former (32,33,40). In my private opinion, definition by volume would be rather complicated and difficult to evaluate for tumors with massive calcification (as depth sometimes cannot be measured). Moreover, the change of volume is consequently greater for originally small tumors than for originally large tumors.
In addition, clear and straightforward evidence about patient-reported outcomes and precise risk stratification would be necessary to encourage health-care professionals all around the world to adopt active surveillance protocols with confidence.
(I) Shared decision-making based on patient-reported outcomes
At CIH, 90% of patients with asymptomatic PMC chose to receive active surveillance rather than immediate surgery between 1995 and 2013. The high rate could be explained by the fact that the author was the only person who informed the protocol of clinical trial to patients at the department. In addition, one quarter of patients had other malignancies. On the other hand, the frequency of using active surveillance was 65% at Kuma Hospital during the period from 1993 to 2016. This frequency gradually increased from 30% in 1993–1997 to 88% in 2014–2016, and varied markedly among individual surgeons (47). Although active surveillance was not equally accepted by all physicians, the management policy is expected to gain relatively rapid acceptance in other hospitals in Japan and around the world, due to increasing evidence of the safety of active surveillance for selected patients with very-low-risk PTC.
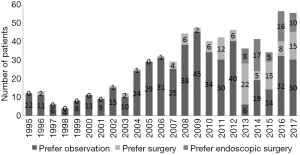
In 2013, the author of this review moved from CIH to the Department of Endocrine Surgery at Nippon Medical School (NMS), an academic hospital in Tokyo, and introduced active surveillance as an option for patients with asymptomatic PMC. The rate of patients who chose active surveillance has gradually been increasing (Figure 6). A total of 101 patients had selected the policy as of 2017. After 1–5 years (mean, 2.4 years) of follow-up, 6 patients (6%) showed tumor size enlargement, 2 patients (2%) demonstrated tumor shrinkage and 93 patients (92%) showed no change, but one patient was revealed to have lymph node metastasis in the lateral neck and underwent surgery 1 year from the beginning of active surveillance. During the same period, 121 patients received immediate surgery at NMS. Among these, 56 patients (46%) preferred to undergo video-assisted endoscopic thyroidectomy, which leaves no scar on the neck and was developed in the department in 1998 (48). To overcome the anxiety of patients diagnosed with “cancer” unexpectedly, we have to provide thorough information about all treatment options based on the scientific evidence. Informed decision-making based on a respect for the autonomy of the patient must be of paramount important to determine the best choice (49). However, studies concerning patient quality of life after making the decision are still lacking (50). Evaluation of patient-reported outcomes from each management option both cross-sectionally and longitudinally are essential to facilitate individual shared decision-making about the care of this indolent disease.
(II) More accurate risk stratification for cT1N0M0 at the time of diagnosis
As described, no definitive predictive factors have been identified to differentiate asymptomatic PMCs that will subsequently progress (type II PMC) from others (type I PMC) before the starting of active surveillance. At present, only patient age seems to offer a reliable indicator for progression of PMC to some extent. However, we cannot end active surveillance even for patients >80 years old or showing tumor with rim calcification, until we acquire evidence regarding when to follow patients. In this aging society, setting up a proper follow-up system with a tracking and reminder program is mandatory to allow lifelong surveillance.
Fortunately, we have never seen the appearance of distant metastasis or cause-specific death during an active surveillance program (29,31-33). However, patients with distant metastasis from an unknown primary origin have occasionally been belatedly revealed to have PMC. Those symptomatic, type III patients uniformly follow an unfavorable course. The process and probability of development from type I PMC to such disease remain unclear. Multi-institutional studies collecting each type of PMC are needed to elucidate these issues. Such studies might need more than hundreds of thousands of type I PMC cases with substantially long follow-up. At CIH and NMS, we routinely conduct lung CT before making a decision for patients with asymptomatic PMC. However, we are not confident regarding how far and how often examinations for distant metastasis should be conducted for those patients under observation.
Management policy for low-risk PTC in Japan
As for PTC, “cancer” that has no deleterious effect on the individual throughout the life of the patient is sometimes present. Japanese pioneers in thyroidology have understood the biological characteristics of the disease and have selected a conservative approach such as lobectomy in an attempt to maintain quality of life for patients with low-risk PTC. Facing the issue of overdiagnosis and overtreatment of very low-risk PTC, our traditional method of conservative treatment yielded active surveillance strategy and is now affecting attitudes toward this disease worldwide.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.10.04). Iwao Sugitani serves as an unpaid editorial board member of Annals of Thyroid from Sep 2017 to Aug 2019.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: Addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004;135:139-48. [Crossref] [PubMed]
- Ebina A, Sugitani I, Fujimoto Y, et al. Risk-adapted management of papillary thyroid carcinoma according to our own risk group classification system: Is thyroid lobectomy the treatment of choice for low-risk patients? Surgery 2014;156:1579-88. [Crossref] [PubMed]
- Takami H, Ito Y, Noguchi H, et al. eds. Treatment of thyroid tumor. Japanese clinical guidelines. Tokyo: Springer, 2010.
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Haigh PI, Ulbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol 2005;12:81-9. [Crossref] [PubMed]
- Mendelsohn AH, Elashoff DA, Abemayor E, et al. Surgery for papillary thyroid carcinoma: Is lobectomy enough? Arch Otolaryngol Head Neck Surg 2010;136:1055-61. [Crossref] [PubMed]
- Barney BM, Hitchcock YJ, Sharma P, et al. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck 2011;33:645-9. [Crossref] [PubMed]
- Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery 2012;151:571-9. [Crossref] [PubMed]
- Ito Y, Masuoka H, Fukushima M, et al. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg 2010;34:1285-90. [Crossref] [PubMed]
- Matsuzu K, Sugino K, Masudo K, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg 2014;38:68-79. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [Crossref] [PubMed]
- Takahashi S. Clinicopathological studies of latent carcinoma of the thyroid. Nihon Naibunpi Gakkai Zasshi 1969;45:65-76. [Crossref] [PubMed]
- Sampson RJ, Key CR, Buncher CR, et al. Papillary carcinoma of the thyroid gland: sizes of 525 tumors found at autopsy in Hiroshima and Nagasaki. Cancer 1970;25:1391-3. [Crossref] [PubMed]
- Fukunaga FH, Yatani R. Geographic pathology of occult thyroid carcinomas. Cancer 1975;36:1095-9. [Crossref] [PubMed]
- Bondeson L, Ljungberg O. Occult thyroid carcinoma at autopsy in Malmö, Sweden. Cancer 1981;47:319-23. [Crossref] [PubMed]
- Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer 1985;56:531-8. [Crossref] [PubMed]
- Furuya-Kanamori L, Bell KJ, Clark J, et al. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis. J Clin Oncol 2016;34:3672-9. [Crossref] [PubMed]
- Takebe K, Date M, Yamamoto Y. Mass screening for thyroid cancer with ultrasonography. Karkinos 1994;7:309-17.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic": Screening and overdiagnosis. N Engl J Med 2014;371:1765-7. [Crossref] [PubMed]
- Guidebook for ultrasound diagnosis of thyroid diseases, Revised 2nd ed. [in Japanese]. Japan Association of Breast & Thyroid Sonology eds. Tokyo, Nankodo, 2012.
- Vaccarella S, Dal Maso L, Laversanne M, et al. The impact of diagnostic changes on the rise in thyroid cancer incidence: A population-based study in selected high-resource countries. Thyroid 2015;25:1127-36. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for thyroid cancer: US Preventive Services Task Force recommendation statement. JAMA 2017;317:1882-7. [Crossref] [PubMed]
- Rosai J. Renaming papillary microcarcinoma of the thyroid gland: The Porto proposal. Int J Surg Pathol 2003;11:249-51. [Crossref] [PubMed]
- Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2:1023-9. [Crossref] [PubMed]
- WHO classification of tumours of endocrine organs, 4th ed. Lloyd RV, Osamura RY, Kloppel G, et al. eds. Lyon, International Agency for Research on Cancer. 2017.
- Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: A retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209-16. [Crossref] [PubMed]
- Sugitani I, Toda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 2010;34:1222-31. [Crossref] [PubMed]
- Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24:27-34. [Crossref] [PubMed]
- Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 2017;143:1015-20. [Crossref] [PubMed]
- Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: A single center’s experience in Korea. J Clin Endocrinol Metab 2017;102:1917-25. [Crossref] [PubMed]
- Oda H, Miyauchi A, Ito Y, et al. Incidence of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid 2016;26:150-5. [Crossref] [PubMed]
- Oda H, Miyauchi A, Ito Y, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J 2017;64:59-64. [Crossref] [PubMed]
- Miyauchi A, Ito Y, Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid 2018;28:23-31. [Crossref] [PubMed]
- Fukuoka O, Sugitani I, Ebina A, et al. Natural history of asymptomatic thyroid microcarcinoma: Time-dependent changes in calcification and vascularity during active surveillance. World J Surg 2016;40:529-37. [Crossref] [PubMed]
- Miyauchi A, Kudo T, Ito Y, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery 2018;163:48-52. [Crossref] [PubMed]
- Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg 2014;38:673-8. [Crossref] [PubMed]
- Kim HI, Jang WJ, Ahn HS, et al. High serum TSH is associated with progression of papillary thyroid microcarcinoma during active surveillance. J Clin Endocrinol Metab 2018;103:446-51. [PubMed]
- Yabuta T, Matsuse M, Hirokawa M, et al. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid 2017;27:1206-7. [Crossref] [PubMed]
- Hirokawa M, Kudo T, Ota H, et al. Pathological characteristics of low-risk papillary thyroid microcarcinoma with progression during active surveillance. Endocr J 2016;63:805-10. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Oda H, et al. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: Was immediate surgery necessary? World J Surg 2016;40:523-8. [Crossref] [PubMed]
- Brito JP, Ito Y, Miyauchi A, et al. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 2016;26:144-9. [Crossref] [PubMed]
- Anderson KL, Youngwirth LM, Scheri RP, et al. T1a versus T1b differentiated thyroid cancers: Do we need to make the distinction? Thyroid 2016;26:1046-52. [Crossref] [PubMed]
- Griffin A, Brito JP, Bahl M, et al. Applying criteria of active surveillance to low-risk papillary thyroid cancer over a decade: How many surgeries and complications can be avoided? Thyroid 2017;27:518-23. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kudo T, et al. Trends in the implementation of active surveillance for low-risk papillary thyroid microcarcinomas at Kuma Hospital: Gradual increase and heterogeneity in the acceptance of this new management option. Thyroid 2018;28:488-95. [Crossref] [PubMed]
- Shimizu K, Akasu S, Tanaka S. Vide-assisted neck surgery: Endoscopic resection of benign thyroid tumor aiming at scarless surgery on the neck. J Surg Oncol 1998;69:178-80. [Crossref] [PubMed]
- Medical Professionalism Project. Medical professionalism in the new millennium: A physicians’ charter. Lancet 2002;359:520-2. [Crossref] [PubMed]
- Davies L, Hendrickson CD, Hanson GS. Experience of US patients who self-identify as having an overdiagnosed thyroid cancer: A qualitative analysis. JAMA Otolaryngol Head Neck Surg 2017;143:663-9. [Crossref] [PubMed]
Cite this article as: Sugitani I. Active surveillance for very low-risk papillary thyroid carcinoma: experience and perspectives from Japan. Ann Thyroid 2018;3:26.


