The evolution and progress of mechanism and prevention of recurrent laryngeal nerve injury
Introduction
Dysfunctions of the recurrent laryngeal nerve (RLN) manifested as nerve paralysis are among the most spectacular, cumbersome and quality of life-deteriorating complications in thyroid and parathyroid surgery. Transient nerve paralysis is estimated to affect on the average approximately 5–6% of all the operated patients, while permanent paralysis is seen in approximately 1% of the subjects. Unfortunately, the above complication rate values continue to refer to specialized centers active in thyroid and parathyroid surgery. Most assuredly, the extent of the problem is much larger than it can be statistically determined, thus, the most important factors that seem to play a significant role in prevention of such complications are continuous education and stressing the importance of laryngeal nerve neuromonitoring in endocrine gland surgery. Starting from the 6th century B.C., through the first documented and supported by evidence references to the role of the RLNs proposed by Galen that were further extended in the of anatomy and illustrations by Leonardo da Vinci and Vesalius and ending with the groundbreaking papers by Kocher, Billroth, Mikulicz and finally Frank Lahey, the history of medicine repeatedly proved the role of the RLNs in the most important function of our larynx, i.e., human voice and its sound and timbre. Believed to date to be the golden standard, visual identification of the RLNs demonstrating their structural integrity is fading into oblivion, making space for the role of intraoperative functional assessment of the nerve and postoperative evaluation of its function. Intermittent intraoperative laryngeal nerve neuromonitoring (I-IONM) based on basic elements of neurophysiology and electromyography, when performed in keeping with the standards of assessing conductivity in the vagus nerve and laryngeal nerve, allows for identifying the nerve, maps its course and establishes the diagnosis of loss of signal (LOS). Being in particular employed in reoperations, local high-stage thyroid tumors or surgery of giant nodular goiter, I-IONM has a disadvantage, i.e., the inherent in the assessment time interval between stimulation and response to the said stimulation. The introduction of continuous intraoperative functional integrity monitoring (C-IONM) has allowed for monitoring LOS in real time, what when combined with knowledge of interpreting electromyographic recordings with respect to signal amplitude and latency has become the most important weapon allowing for an immediate reaction preventing nerve injury. All the above presented advantages of intraoperative laryngeal nerve neuromonitoring would not be possible without a body of knowledge encompassing descriptive anatomy of the neck, nerve structure, possible etiology of injuries and interpretation of electromyographic recordings combined with the ability to solve problems. Thus, the words of Charles Higgins published in the Annals of Surgery in 1927: “…The prevention of injuries of the recurrent laryngeal nerve demands an accurate understanding of the anatomical relations of the nerve on the part of everyone who deals with surgery of the larynx or of the thyroid gland; the fact that anomalies of the nerve may occur must be constantly kept in mind, the patient who has suffered an injury to the recurrent nerve must always be kept under observation in order to prevent an unfortunate sequel…” acquire particular significance (1-3).
Clinical anatomy of the RLN
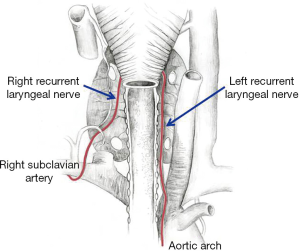
Understanding the topographic anatomy of the nerves crossing the tracheal area is a prerequisite of identification and protection of these life-important structures. Approximately around the 5th week of gestational life, the vagus nerve develops, and at the end of the 6th week, a branch is formed that will later initiate the development of the RLN. The branch, associated with the sixth pharyngeal arch that builds the embryonic germs of the pharynx and larynx, in combination with the fourth germ responsible for forming the main arteries (e.g., aortic arch, right subclavian artery, pulmonary arteries) becomes gradually elongated as the embryo develops. The larynx is relocated upward, while the aortic arch and the associated vessels remain in the chest together with the vagus nerve branch. The branch forms a characteristic recurrent loop called the RLN. In view of the asymmetric positioning of the great vessels on the right and left side of the body, initially, the right and left nerve have different courses. The right nerve turns anteriorly and extends posteriorly around the subclavian artery, while the left nerve goes around the aortic arch; this is a detail that is helpful in identification and finding not only the said nerve, but also the vagus nerve and the situated medially ligamentum arteriosum. Although the further course of the two nerves seems to be similar, it should be remembered that at the right side, the nerve forms an angle extending towards the tracheoesophageal groove to reach the larynx, while on the left side, the nerve extends almost parallelly to promptly fit in the aforementioned groove (Figure 1).
Developmental anomalies resulting in development of a non-RLN on the right side (0.5% to 1%) are associated with lack of formation of the fourth pharyngeal arch, leading in consequence to the right subclavian artery branching off the aortic arch on the left side. The right laryngeal nerve is not stretched as the neck elongates and the heart descends into the chest cavity. Also in this case we need to have knowledge on possible locations of the non-RLN with respect to the inferior thyroid artery (ITA) that supplies the thyroid gland:
- Type I—a high takeoff from the vagus and a descending course running with superior thyroid vessels;
- Type IIa—a lower takeoff from the vagus and a more horizontal course, parallel to the ITA trunk;
- Type IIb—a lower takeoff from the vagus and a more ascending course, running inferiorly to the ITA trunk.
On the left side, the non-RLN is encountered extremally rarely (0.04%) and is concomitant with other congenital malformations, such as situs in vertus, abnormal subclavian artery and absence of ductus arteriosus (4-8).
As it has been already mentioned, an extremely important element of the topographic anatomy of the RLN is its location with respect to the ITA. In their analysis performed in corpses, Campos et al. described numerous variants of the mutual course of the two structures, basically distinguishing three groups, although having various representations depending on the examined side:
- RLN may pass between the branches of the ITA: right (50%); left (35%);
- RLN may pass posterior to the branches of the ITA: right (25%); left (50%);
- RLN may pass anterior to the branches of the ITA: right (25%). left (15%).
In summary, in as many as 62.68% of cases, the relations observed on one side were not repeated on the opposite side. The complexity of the relations is illustrated in the publication by Ozgüner et al. who distinguished as many as seven types of mutual positioning of the ITA and the RLN:
- Type 1: RLN situated posterior to the artery; right (42.5%), left (65%);
- Type 2: RLN situated anterior to the artery; right (40.5%), left (22.5%);
- Type 3: RLN situated parallel to the artery; right (11.5%), left (7%);
- Type 4: RLN situated between the two branches of the artery; right (1%), left (3.5%);
- Type 5: The extralaryngeal branch of RLN was detected before it crossed ITA; right (4.5%), left (0%);
- Type 6: ITA situated between the two branches of RLN; right (0%), left (0.5%);
- Type 7: the branches of RLN situated among the branches of ITA; right (0%), left (0.5%).
Summing up the above information, it should be said that the nerve is always in the vicinity of the artery, but the fact should not affect the unambiguous determination of the location and course of the nerve with respect to the artery (9-11).
While pondering on understanding the topography of the RLN with respect to the surrounding structures, its mutual correlations with the Zukerkandl’s tubercle and Berry’s ligament should be mentioned. In the first case, one should remember about the position of the nerve between the tubercle and the tracheal wall, in the other about the structure of the ligament itself. If the identified nerve has a small diameter, it is recommended to leave a small, devoid of lesions remnant of the thyroid tissue in the vicinity of the nerve. Until recently, the knowledge of anatomy combined with visual identification of nerves during surgery was the standard of management in thyroid and parathyroid surgery. The genuine revolution that occurred in the past few years is the intraoperative neuromonitoring (IONM) of the laryngeal and vagus nerves during neck-involving procedures. The introduction of the new technique has allowed for a better understanding of mechanisms of injuring the nerve structures, simultaneously emphatically showing that the anatomical continuity of the exposed nerve does not always go parallel to its functional integrity, at the same time providing the surgeon with an opportunity to modify the surgical technique in order to protect the nerves visible in the surgical field (12).
Diagnosis of RLN injury based on IONM
The introduction of IONM in thyroid and parathyroid surgery has facilitated not only nerve identification and localization of injuries, but most of all, it has allowed for confirming the concordance of functional and visual localization and paved the road for investigations aiming at understanding the mechanisms of nerve injury. Thus, the IONM technology has significantly affected numerous aspects of thyroid and parathyroid surgery, starting from advantages apparent in daily clinical practice, research and education and ending with medico-legal issues involved in the procedures performed. Looking from the perspective of the last two decades and numerous reports addressing the problem, one should undoubtedly stress the fact that only accepting the standardized method proposed by the International Neural Monitoring Study Group and based on the four-stage procedure has provided an opportunity to achieve comparable and true results and become an element of not only the learning process, but also of formulating further recommendations. The procedure, based on intraoperative analysis of the electromyographic signal (EMG) from the vagus (V signal) and laryngeal (R signal) nerves prior to and after the gland resection and on the laryngological assessment according to the scheme L1-V1-R1-R2-V2-L2 has provided the complete picture of the nerve functional integrity, thus allowing for (13-15):
- Postoperative prognostication of vocal cords function;
- Introduction of the procedure preventing bilateral injuries of the RLNs, the so-called “stage thyroidectomy”;
- Localization of conductivity disturbances and identification of the injury site or character, especially when employing continuous neuromonitoring (C-IONM);
- Identification and early differentiation between changes of voice that are dependent or independent from intraoperative injuries of the RLN (RLN-related and unrelated);
- Differentiation of anatomical variants.
Etiology of vocal fold paralysis
The analysis of the literature published in the last two decades and addressing surgery of the head, neck and chest points to a definite reversal in the etiology of vocal fold paralysis. Extrathyroidal (67%) reasons for surgical procedures have gained primacy of being the most significant in unilateral vocal folds paralysis, while thyroid surgery continues to play a dominating role as the most common cause of bilateral paralysis (80%). Surgery (37%) continues to be one of the most common causes of vocal fold paralysis; nevertheless, one should bear in mind other causes, such as idiopathic (19.6%), non-surgical iatrogenic (11.1%), neurological (7.9%), intubation-related (7.5%), cardiogenic (4.3%) and very rare cases of infectious causes (tbc) (1.1%) (16-19). Table 1 presents the most frequent iatrogenic causes of vocal fold paralysis.
Table 1
| Latrogenic—surgical |
| Neck surgery |
| Thyroid (26%) and parathyroid (7%) surgery |
| Anterior cervical spine approach—most often right side (15%) |
| Carotid endarterectomy (11%) |
| Implantation of vagal nerve stimulator |
| Zenker’s diverticulum repair |
| Parapharyngeal tumor resection, e.g., vagal schwannoma, neurofibroma, carotid body tumor |
| Radical neck dissection |
| Lymph node excision from supraclavicular region |
| Thoracic surgery |
| Pulmonectomy and other lobectomies |
| Thoracotomy and tracheal surgery |
| Thymectomy |
| Cardiac surgeries (9%): ductus arteriosus ligation, coronary artery bypass graft, aortic valve replacement, tetralogy of Fallot repair, cardiac and pulmonary transplant |
| Mediastinoscopy |
| Esophageal surgery |
| Thoracic aortic aneurysm repair |
| Head surgery |
| Skull base surgery (foramen magnum decompression) |
| Intracranial aneurysm |
| Latrogenic—non-surgical |
| Endotracheal intubation |
| Non-iatrogenic |
| Blunt or penetrating trauma of the neck |
| Others |
| Central venous catheterization |
The mechanism of RLN injury
Intraoperative injury of the RLN often results from failure to follow the principles of proper surgical technique what results in nerve conduction disorders while the nerve anatomical integrity is maintained. The most common causes include unintentional maneuvers leading to excessive traction and thus stretching the nerve, a mechanical damage resulting from compression, contusion or external pressure, the effect of high temperatures in the vicinity of the nerve producing its thermal damage, and finally ischemia, clamping or transection. The most frequent causes are listed in Table 2. In their report, Dionigi et al. listed the principal reasons as “…traction (71%), thermal (17%), compression (4.2%), clamping (3.4%), ligature entrapment (1.6%), suction (1.4%), and nerve transection (1.4%). Complete recovery from vocal cord palsy (VCP) was documented in 91% of RLNIs. Recovery time was significantly faster in the traction group compared to the other groups (P<0.001). The rates of temporary and permanent VCP were 98.6 and 1.4% for traction lesion, 72 and 28% for thermal injury, 100 and 0% for compression injury, 50 and 50% for clamping injury, 100 and 0% for ligature entrapment, 100 and 0% for suction injury, and 0 and 100% for nerve transection, respectively…”. When we read the entire text we realize that the most important message of the publication—apart from the knowledge and documentation of genuine dangers—is the fact that approximately 86% injuries of the RLN were detected thanks to the use of IONM in spite of the fact that no anatomical changes in the nerve integrity were seen. Understanding the histological structure of the axon projections supported by the knowledge on conduction physiology have served for formulating two pathophysiological classifications of degrees of nerve injury. The said systems were developed by Seddon and Sunderland; they were based on understanding the nerve fiber morphology and systematized the histological knowledge on the depth of injury, thus introducing an important prognostic factor that was dependent on the degree of nerve injury (Table 3). The above classification, although it provided a formidable explantation of nerve injuries and their consequences, could not have been clinically useful. Only the introduction of electrophysiological classification proposed by Chiang et al. did allow for dividing nerve injuries into two basic types (20,21):
Table 2
| Transection (intentional or inadvertent) |
| Constricting and inadvertent clamping injury: tying or clamping a vessel too closely, bifurcated RLN, hemostasis at the region of Berry’s ligament |
| Stretch and traction |
| Thermal injury (energy-based devices) |
| Compression, contusion, suction, pressure |
| Ligature |
| Ischemia |
RLN, recurrent laryngeal nerve.
Table 3
| Seddon classification | Sunderland classification | Pathophysiologic results | Prognosis |
|---|---|---|---|
| Neurapraxia | Type 1 | Interruption of impulse conduction in an axon, without anatomic disruption. (compression or ischemia) | Full recovery in days up to months |
| Axonotmesis | Type 2 | The endoneurial tube (endoneurium, perineurium, epineurium intact) remains intact, but the axon loses continuity | Rather good but slow |
| Type3 | Loss of axon and endoneurium continuity, the intrafascicular structure is disorganized | Incomplete—the regenerating axons reinnervate the wrong target or a scar within the fascicle can block axonal regeneration | |
| Type 4 | The fascicles are disrupted (loss of axon, endoneurium, perineurium) | Complete disorganization of regenerating axons; prognosis only after repair (the role of graft materials) | |
| Neurotmesis | Type 5 | The nerve trunk is completely transected | There is no regeneration; prognosis only after repair (the role of graft materials) |
- Type 1 injury segmental/localized RLN injury: strict and fast direct distress on the nerve. Caused by traction (68%), thermal injury (16%) and sometimes pinching (13%);
- Type 2 injury diffuse or global, non-specific disruption point and no visible change of the nerve caused by traction (92%) (the site of injury is usually at a higher position above the nerve entry to the larynx).
Prognostic factors of RLN injury
The use of neuromonitoring of the RLNs, although initially produced a considerable number of false results, in consequence provided surgeons with a tool for better understanding of hitherto incomprehensible phenomena of neural conductivity and paved the road to further investigations. Visual nerve identification combined with recording of functional electromyographic activity have allowed for improvement of surgical techniques aiming at preventing primary laryngeal nerve injuries, become an important element in postoperative prognosing the effects of injury and affected the change in surgical strategy in case of intraoperative LOS. All these factors have exerted an impact on improvement of quality of life and allowed for further development of counselling and therapy of patients suffering from vocal fold paralysis. Based on an extensive material (281 nerve injuries in the group of 6,093 nerves at risk), Dionigi et al. assessed the surgical risk and functional recovery of the injured nerves (22). Thanks to their studies performed in an animal model and correlation with clinical data, they proposed a new system of classification of the degree of intensity and function recovery of the injured nerves depending on the mechanism of injury:
- Traction injuries (Class-A1: traction at ligament of Berry-LOB region; Class-A2: traction at goiter adhesion) (Type 1 focal segmental electrical defect can be detected at the region of LOB, and Type 2 focal segmental electrical defect cannot be detected on the exposed RLN) were defined as injuries of mild severity with the short recovery time (27±9 days), low rate of permanent RLN palsy (1.4%), physical changes (5%), and mild histological disturbances;
- Mechanical trauma (Class-B 1: RLN is injured by blunt force, compression, contusion, pressure or suction; Class B2: RLN is injured during dissection by surgical instruments);
- Constriction: (ligature, clipping of the small blood vessels, and formation of constricting band around nerve tissue); both of them were defined as injuries of mild or moderate severity;
- Thermal injuries (Class-D): RLN is injured by high temperature of electrocautery or energy-based device; this damage was defined as injuries of moderate or high severity: lower recovery time (91±11 days), higher rate of permanent RLN palsy (28%), physical changes (22%). This type of RLN injury is much more dangerous for the nerves at risk;
- Transection injuries (Class-E): RLN is completely cut during the dissection around soft tissue.
Based on data from the literature on the subject, the most common causes of laryngeal nerve injuries include traction, constriction, transection and thermal injury, which undoubtedly occur in the course of thyroidectomy. Early RLN location and identification, protection from high temperature by keeping adequate distance and time of exposure and mild traction of the thyroid tissue in the course of the surgical procedure are the most important prerequisites of a proper postoperative course.
In the last few years we observed dynamic progress a new technique of IONM continuous neuromonitoring (C-IONM). This technique compare to intermittent neural monitoring is much better and improve to detect risk of injury of the RLN during a real time operation amplitude and latency of vagal nerve. Despite new abilities the implementation of the new technique requires precise preoperative evaluation recognizes RLN malfunctions before surgery, the ability to solve problems based on knowledge of not only the technique but also the physiology of nerve transmission and accurate intraoperative management avoiding confounding intraoperative factors.
Conclusions
Significant progress occurring over the past 20 years in monitoring quality of life after thyroid and parathyroid surgery would not be possible without the development of modern surgical techniques and improvement of devices, but we always remember that lower complication rate depends on experience operating team, accurate technical skill and surgeon’s knowledge about solve the problems during thyroid and parathyroid surgery. The introduction of IONM of the RLNs has fundamentally affected the improvement in the quality of surgical procedures, limiting the number of complications and improving quality of life (23).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcin Barczyński, Hui Sun, and Xiaoli Liu) for the series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.11.02). The series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” was commissioned by the editorial office without any funding or sponsorship. Marcin Barczyński served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Thyroid from Aug 2017 to Jul 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Higgins CC. Surgical anatomy of the recurrent laryngeal nerve with especial reference to thyroid surgery. Ann surg 1927;85:827-38. [Crossref] [PubMed]
- Kark AE, Kissin MW, Auerbach R, et al. Voice changes after thyroidectomy: role of the external laryngeal nerve. Br Med J (Clin Res Ed) 1984;289:1412-5. [Crossref] [PubMed]
- Zeiger MA, Shen WT, Felger EA. The Supreme Triumph of the Surgeon's Art: Narrative History of Endocrine Surgery. San Francisco: University of California Medical Humanities Press, 2013:104-16.
- Kamani D, Potenza AS, Cernea CR, et al. The nonrecurrent laryngeal nerve: anatomic and electrophysiologic algorithm for reliable identification. Laryngoscope 2015;125:503-8. [Crossref] [PubMed]
- Kandil E, Anwar MA, Bamford J, et al. Electrophysiological identification of nonrecurrent laryngeal nerves. Laryngoscope 2017;127:2189-93. [Crossref] [PubMed]
- Henry JF, Audiffret J, Denizot A, et al. The nonrecurrent inferior laryngeal nerve: review of 33 cases, including two on the left side. Surgery 1988;104:977-84. [PubMed]
- Fellmer PT, Böhner H, Wolf A, et al. A left nonrecurrent inferior laryngeal nerve in a patient with right-sided aorta, truncus arteriosus communis, and an aberrant left innominate artery. Thyroid 2008;18:647-9. [Crossref] [PubMed]
- Skandalakis JE, Droulias C, Harlaftis N, et al. The recurrent laryngeal nerve. Am Surg 1976;42:629-34. [PubMed]
- Campos BA, Henriques PR. Relationship between the recurrent laryngeal nerve and the inferior thyroid artery: a study in corpses. Rev Hosp Clin Fac Med Sao Paulo 2000;55:195-200. [Crossref] [PubMed]
- Ozgüner G, Sulak O. Arterial supply to the thyroid gland and the relationship between the recurrent laryngeal nerve and the inferior thyroid artery in human fetal cadavers. Clin Anat 2014;27:1185-92. [Crossref] [PubMed]
- Chiang FY, Lu IC, Chen HC, et al. Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci 2010;26:575-83. [Crossref] [PubMed]
- Randolph GW, Dralle H, Abdullah H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123:S1-14. [Crossref] [PubMed]
- Dionigi G, Barczynski M, Chiang FY, et al. Why monitor the recurrent laryngeal nerve in thyroid surgery? J Endocrinol Invest 2010;33:819-22. [Crossref] [PubMed]
- Barczyński M, Konturek A, Stopa M, et al. Total thyroidectomy for benign thyroid disease: is it really worthwhile? Ann Surg 2011;254:724-29; discussion 729-30. [Crossref] [PubMed]
- Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. Laryngoscope 2007;117:1864-70. [Crossref] [PubMed]
- Benninger MS, Gillen JB, Altman JS. Changing etiology of vocal fold immobility. Laryngoscope 1998;108:1346-50. [Crossref] [PubMed]
- Apfelbaum RI, Kriskovich MD, Haller JR. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976) 2000;25:2906-12. [Crossref] [PubMed]
- Gokaslan ZL, Bydon M, De la Garza-Ramos R, et al. Recurrent Laryngeal Nerve Palsy After Cervical Spine Surgery: A Multicenter AOSpine Clinical Research Network Study. Global Spine J 2017;7:53S-57S. [Crossref] [PubMed]
- Chiang FY, Lee KW, Chen HC, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 2010;34:223-9. [Crossref] [PubMed]
- Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. [Crossref] [PubMed]
- Dionigi G, Wu CW, Kim HY, et al. Severity of Recurrent Laryngeal Nerve Injuries in Thyroid Surgery. World J Surg 2016;40:1373-81. [Crossref] [PubMed]
- Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009;96:240-6. [Crossref] [PubMed]
Cite this article as: Konturek A, Barczyński M. The evolution and progress of mechanism and prevention of recurrent laryngeal nerve injury. Ann Thyroid 2018;3:32.