
Thyroid oncology in pregnancy
Introduction
Thyroid disorders in young women of reproductive age are common; more than 11% of them have autoimmune thyroid diseases, 3% have hypothyroidism and 2% have hyperthyroidism (1). The incidence of thyroid cancer—the most common endocrine malignancy—has been rising in the last few decades, and often affects women between 20 and 39 years of age. Thyroid cancer is the second most commonly diagnosed malignancy during pregnancy (after breast cancer), with 14 cases per 100,000 pregnancies. It is always is a huge challenge for clinicians, requiring special consideration and attention for both the patient and her developing child (2). On the one hand, the main protocols for diagnosing and treating thyroid cancer in pregnancy are the same as in cases diagnosed in the rest of the population, with some restrictions [e.g., administration of radioactive iodine (RAI) as a part of therapy is forbidden] (3,4). On the other hand, “there is always a conflict between maternal optimal therapy and fetal well-being” according to Oducan’s review of maternal-fetal issues related to malignancies in pregnant women (5). There are many aspects that should be discussed regarding thyroid malignancy during pregnancy, such as the timing of surgery, systemic therapy for thyroid cancer during pregnancy and the effect of pregnancy on thyroid cancer outcomes. The influence of RAI ablative therapy and therapy with levothyroxine (LT4) after thyroid cancer treatment should be considered in relation to possible future pregnancies.
The aim of this study was to evaluate the recent literature and guidelines regarding thyroid malignancy in pregnancy, and to provide answers to these questions.
Changes in thyroid physiology during pregnancy
During pregnancy, physiological changes in the maternal thyroid hormones are observed: thyroid-stimulating hormone (TSH) levels decrease in the first trimester and then return to normal before delivery. This is due to high levels of β-human chorionic gonadotropin (β-hCG) produced by the placenta, which stimulates production of thyroid hormones by cross-reacting with TSH-receptors, increasing the activity of the maternal thyroid gland (6,7). Both TSH and β-hCG are glycoprotein hormones encoded by the same gene (8). This leads to suppression of maternal TSH secretion through negative feedback mechanisms, and the increased production of thyroid hormones via β-hCG stimulation results in elevated levels of free T4 and free T3. Throughout pregnancy, the range of TSH secretion is lower than the reference levels for non-pregnant women. Due to the transfer of thyroid hormones to the fetus and increased maternal thyroxine-binding globulin (TBG) stimulated by high estrogen levels, maternal thyroid hormone requirements increase up to 50% during gestation (9). In the second and third trimesters of pregnancy, thyroid hormone secretion returns to normal when β-hCG levels decrease.
Knowledge of fetal thyroid development is equally important. At approximately 10 weeks of gestation, small amounts of thyroid hormone are produced by the fetal thyroid gland, with progression at about 35 weeks. It follows that in the first trimester of pregnancy, the fetus is totally dependent on the transfer of thyroid hormones from the mother (10). Precise regulation of thyroid hormone levels during gestation is crucial to achieve optimal outcomes for both the mother and child. Maternal hypothyroidism is one of the most common complications of pregnancy, increasing the risk of miscarriage, preeclampsia, neurologic and congenital impairment or abnormal fetal growth (3,11). While euthyroid pregnant women are able to compensate for all the physiological changes during gestation, it is strongly recommended for overt hypothyroidism to be treated during pregnancy (3,7).
The relationship between estradiol, β-hCG and the thyroid gland is under investigation by many researchers as a potential risk factor for thyroid malignancy in pregnancy (3,12-14). The meta-analysis in Mannathazhathu et al. supports an association due to changes in female hormones during menstrual cycle and pregnancy with the risk of thyroid carcinoma (TC) and explains female preponderance (15).
Epidemiology of thyroid cancer and pregnancy
Thyroid cancer is the most common malignancy among endocrine disorders, accounting for 3.6% of all malignant tumors. During pregnancy, thyroid cancer is the second most common malignancy (after breast cancer), with a rate of 14 per 100,000 live births; papillary thyroid carcinoma (PTC) is the most frequent pathological type. The time of diagnosis has been reported as follows: 3.3/100,000 cases are diagnosed before delivery; 0.3/100,000 are diagnosed at delivery; and 10.8/100,000 within 1 year post-partum (2,3). Further, it is known that there is a high frequency of co-occurrence of differentiated thyroid cancer (DTC) and breast cancer (4) Easy access to modern diagnostic tools, particularly to ultrasound examination, could be one of the reasons why the incidence of thyroid cancer has increased in recent decades all over the world (16,17). Increases observed in some geographical regions in Eastern Europe are associated with radioactive fallout from the Chernobyl nuclear power station accident (18).
The incidence of thyroid cancer generally increases with age, and it is more common in females than in males; the female to male ratio is 3:1 (3). The predominance among females suggests that hormonal factors may be involved. Some studies suggest that biological changes during pregnancy may increase the risk of TC (12-14,19-21); but others show no significant correlation between pregnancy and the risk of thyroid malignancy (22,23). A study by Horn-Ross et al. found that for woman under 45 years old, the risk of thyroid cancer increased in the first 5 years following pregnancy, again suggesting that pregnancy could be a potential risk factor for TC. Moreover, in women older than 45 years old, the use of estrogen replacement therapy was associated with a trend toward an increased risk of PTC (21). In contrast, a population-based retrospective study by Mack et al. concluded that the risk of thyroid cancer was not altered by the use of estrogens (oral contraceptive pills). Interestingly, they found a correlation between the prevalence of thyroid cancer and lactation: in women who suppressed lactation post-partum they observed an increased incidence of cancer, while the rate of thyroid cancer decreased among women who had ever breastfed; and a longer duration of lactation was associated with lower incidence of cancer (22). In another big-cohort study, including 117,646 women, the authors showed an association between a late age of menarche, a longer menstrual cycle (more than 30 days) and an increased risk of thyroid cancer (24). One of the biggest meta-analyses, including 21 studies with 406,329 cases, showed a strong association between parity (>3 pregnancies) and the risk of thyroid cancer.
Thyroid nodules during pregnancy
Thyroid cancer is usually diagnosed within a thyroid nodule. The high level of β-hCG and estrogens and the negative iodine balance during pregnancy causes up to 30% growth not only of the thyroid gland, but also in preexisting thyroid nodules. Moreover, all these changes stimulate the formation of new thyroid nodules (25,26). In most cases, the increased nodule volume returns to pre-pregnancy diameter 3 months post-partum (27). According to American College of Obstetricians and Gynecologists, if a nodule is present during the first trimester of pregnancy, up to 20% of women will develop a second nodule during the same pregnancy (26). According to American Thyroid Association (ATA) recommendations, any thyroid nodule recognized during pregnancy should undergo the same evaluation as a nodule detected in any other patient (3). This means that patients should be asked about their personal and family history of TC, about multiple endocrine neoplasia type 2 (MEN2) and familial adenomatous polyposis (FAP). Any history of neck irradiation, compressive symptoms, dysphagia or dysphonia should be taken under consideration in the diagnosis (3,28). In all nodules suspicious for malignancy or in nodules greater than 1 cm in diameter, a fine need aspiration (FNA) biopsy should be performed. This can be done at any time during pregnancy; there is no risk for either the mother or the fetus (29). Some authors suggest that the FNA could be delayed if the nodule is recognized late in the pregnancy or if treatment is postponed until after delivery, assuming that an ultrasound exam reveals no signs of malignancy (29,30).
TSH measurement should be performed on any thyroid nodule recognized in a pregnant woman. It is important to be careful with TSH interpretation. Subnormal serum TSH in non-pregnant women suggests a functioning nodule, but in pregnancy it could be a physiological change (3). A serum calcitonin measurement is required only in pregnant women with a history of medullary TC, MEN2 or known RET gene mutations. Pentagastrin stimulation test is contraindicated in pregnancy (3,30,31); serum TG measurement is not recommended either (26). Finally, RAI scans and scintigraphy with either technetium-99m or iodine-131 (I-131) are strictly contraindicated during pregnancy, because I-131 crosses the placenta (32).
To sum up, the diagnosis of a thyroid nodule during pregnancy is in most cases based on cancer risk stratification, including clinical examination, history, ultrasonography, serum TSH measurement and FNA.
Treatment of thyroid cancer and timing of surgery
Total thyroidectomy is the treatment of choice in thyroid malignancy (3), but in pregnancy, both maternal and fetal outcomes need to be taken under consideration before making decisions about surgery. According to the ATA and the Endocrine Society, in most cases a thyroidectomy can be delayed until the post-partum period (3,33). In the treatment of PTC and follicular thyroid cancer (FTC), there is no evidence of worse outcomes when surgical treatment is delayed several months (Figure 1) (3). If thyroid cancer is diagnosed in the first trimester, it is necessary to monitor the lesion by regular neck ultrasound examinations in each trimester. Usually tumors remain stable, but if significant growth is observed (i.e., more than 50% in volume and more than 20% in diameter) or if lymph node metastases are observed, surgery is recommended.
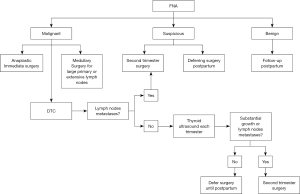
If the thyroid cancer is recognized in the second or third trimester, the operation should be delayed until after delivery, except in cases where advanced disease is observed. If bulky cervical adenopathy is observed, confirming advanced disease, the thyroid operation should be performed as soon as possible (3,6,32,33). The optimal timing for surgery in pregnant women is the second trimester, when organogenesis is completed, but the rate of complication is always higher in pregnant women than in non-pregnant patients (3,30-33). In an analysis involving 201 pregnant women who underwent thyroid and parathyroid operations, Kuy et al. found that they were twice as likely as non-pregnant controls (over 31,000) to experience complications. There are many risk factors for complications in pregnant women (34). An enlarged thyroid gland makes surgery more difficult, and anesthesia is more complicated because of gestational physiological changes. The most dangerous for both the mother and fetus seems to be the first trimester, with the risk of spontaneous abortion (3,5,33); whenever it is possible, first-trimester surgery should be avoided.
In all cases of thyroid malignancy recognized during pregnancy, suppressive doses of LT4 are recommended, maintaining optimal TSH levels between 0.1–0.5 mIU/L. Suppressive treatment is not recommended in suspicions of malignancy diagnosed by FNA, because in the literature up to 70% of such tumors are benign (35). Appropriate LT4 supplementation is necessary following a thyroidectomy to prevent maternal and fetal hypothyroidism. The goal is to maintain maternal TSH <2.5 mIU/L during the first trimester and <3.0 mIU/L during the second and third trimesters. Serum TSH levels should be measured every 4 weeks until the end of the pregnancy.
The ATA and the Endocrine Society have established guidelines for using radioactive I-131 therapy in some cases of DTC. In pregnant women I-131 is always contraindicated due to the risk of exposing the fetus to radioactivity. Exposure to RAI during gestation causes fetal hypothyroidism, cognitive disorders and mental retardation. If RAI is indicated it should be recommended as soon as possible post-partum, and breast feeding should be stopped at least 6 weeks before ablation therapy (14,36).
Outcomes of thyroid cancer during pregnancy
Usually the treatment of thyroid cancer diagnosed during pregnancy is delayed until after delivery, which raises the question of whether the postponement causes greater morbidity and mortality. According to many studies, it seems that the diagnosis of thyroid cancer during pregnancy and the frequent delay of surgery does not have a significant impact on the prognosis and outcomes (37-39). Moosa et al. compared the outcomes of DTC in women diagnosed during pregnancy with those diagnosed outside pregnancy. In their study, 77% of the pregnant women with carcinoma underwent surgery post-partum; 20% had their surgery in the second trimester. The 20 years of follow-up included recurrences of cancer, distant metastases and cancer deaths; the outcomes for women with TC diagnosed during pregnancy were similar to those for women diagnosed post-partum. Moreover, there were no differences in the results of treatment among pregnant women operated on in the second trimester as opposed to postpartum (37). A study by Herzon et al. found similar results: among pregnant women aged 18–46 with recognized TC, the survival rates were similar to those of women with carcinoma diagnosed post-partum (38). These results were confirmed by Yasmeen et al., whose study compared thyroid cancer prognoses and maternal and fetal outcomes among pregnant and non-pregnant groups. They found no significant differences between these groups in terms of thyroid cancer prognoses or in any of the maternal or fetal outcomes measured (39). Finally, neither the timing of the thyroid cancer diagnosis, nor the timing of the initial thyroidectomy influence maternal or fetal outcomes. The decision to delay thyroidectomy in DTC during pregnancy is an acceptable option with no impact on outcomes.
Another important question related to malignancy and pregnancy is whether pregnancy alters the course of DTC. Could women who become pregnant after treatment for DTC be at increased risk of disease progression and recurrence? A retrospective study by Hirsch et al. found that among women without any structural or biochemical evidence of DTC at the time of conception, pregnancy did not increase the risk of recurrence during a 4-year postpartum follow-up period. Despite these findings, disease progression might always occur during pregnancy (33). In the retrospective study by Rakhlin et al.’s there is a conclusion that none of the patients with an excellent, indeterminate or biochemical incomplete response to therapy prior to pregnancy developed structurally identifiable disease after a full-term delivery. Even if progression was seen in almost a third of the patients with structural disease prior to pregnancy, only a minority of these patients had changes sufficient to warrant additional therapy. The study confirmed that pre-pregnancy response to therapy status is an excellent predictor of pregnancy-associated disease progression in women previously treated for DTC (40-43). Pregnancy outcomes after thyroid cancer was evaluated in Spiegel et al.’s study (44). Interestingly neonates of mothers with thyroid cancer were not found to be at increased risk for the adverse neonatal outcomes examined, specifically, congenital malformations, intrauterine growth restriction, fetal death and preterm labor (44).
To sum up, the history of thyroid cancer does not meaningfully impact the risk of adverse pregnancy outcomes (41-44). Clinicians usually advise avoiding pregnancy for 12 months after RAI treatment to ensure remission (40,45).
The impact of I-131 treatment of DTC on fertility, pregnancy and breastfeeding
There are only a few publications investigating the impact of I-131 therapy for DTC on fertility, pregnancy and breastfeeding. Bal et al. evaluated 40 women who conceived 7–12 months after RAI ablation of DTC. According to that study, RAI therapy had no effect on fertility, pregnancy complications such as miscarriage, or the health of the offspring, even in mothers treated with large doses of I-131 and in women who conceived less than 12 months after RAI treatment (46). Despite the slight risk that RAI therapy could impact a future pregnancy, the ATA recommends delaying pregnancy until 6 months to 1 year following I-131 (3,46). It is known that RAI ablation treatment can adversely affect gonads in males and females. There are some studies aiming to determine ovary damage and infertility risk due to RAI, using serum anti-Müllerian hormone (AMH) level, in females who received RAI ablation treatment (47-51). Acibucu et al. in his study, compered female patients who have not gone through the menopause and had received RAI ablation treatment for well-DTC in premenopausal period with healthy females as control groups (47). In this study no statistically significant relation between RAI exposure duration and AMH levels was determined. According to these studies, it may be concluded that low AMH levels due to RAI treatment can cause damage to the ovaries of patients; nevertheless, considering the AMH levels and the absence of infertility in the patients, the infertility risk was found to be low (47). Similar results we found in Giust et al. and Mittica et al. and studies; they conclude that nowdays the age is the only predictor of AMH levels (48,49). While Yaish et al. pointed that RAI in DTC has a rapid and profound effect on ovarian reserve, with only a partial recovery potential. In an era of declining human fertility, it is of relevance to recognize the potentially adverse effect of RAI in women of reproductive age (50). Also, Evranos et al.’s study confirmed that AMH is considered an important marker of ovarian reserve. But this study shows that ovarian reserve decreased after RAI therapy (51).
The RAI therapy should also be considered to breastfeeding. Because of the transfer of radioiodine to human breast milk, it is recommended that women avoid breastfeeding for 6 months to 1 year following I-131 treatment (3).
Conclusions
The treatment of thyroid malignancy in pregnancy is a challenge for physicians and requires a multi-disciplinary approach, including the patient’s obstetrician, an endocrinologist, an endocrine surgeon, a nuclear medicine specialist and the baby’s pediatrician. The treatment of choice for DTC—thyroidectomy followed by RAI ablation—poses a significant risk for both the mother and the fetus, and in most cases it can be delayed until post-partum without any negative impact on either maternal or fetal outcomes. If surgical treatment is necessary during pregnancy due to aggressive thyroid malignancy, the optimal time for surgery is the second trimester. RAI therapy, however, is contraindicated during pregnancy. The outcomes of DTC in pregnancy do not usually differ from the rest of the population with thyroid malignancy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Thyroid for series “Recent Challenges in the Management of Thyroid Tumors”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-2020-rcmtt-02). The series “Recent Challenges in the Management of Thyroid Tumors” was commissioned by the editorial office without any funding or sponsorship. MB served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Thyroid from Oct 2019 to Sep 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99. [Crossref] [PubMed]
- Smith LH, Danielsen B, Allen ME, et al. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol 2003;189:1128-35. [Crossref] [PubMed]
- Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017;27:315-89. [Crossref] [PubMed]
- Kurata A. Differentiated thyroid cancer: why does it affect predominantly women during the reproductive period and have higher incidence of mutual association with breast cancer? Med Hypotheses 2019;122:5-7. [Crossref] [PubMed]
- Oduncu FS, Kimmig R, Hepp H, et al. Cancer in pregnancy: maternal-fetal conflict. J Cancer Res Clin Oncol 2003;129:133-46. [Crossref] [PubMed]
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2543-65. [Crossref] [PubMed]
- Dong A, Stephenson M, Stagnaro-Green S. The need for dynamic clinical guidelines: a systematic review of new research published after release of the 2017 ATA guidelines on thyroid disease during pregnancy and the postpartum. front. Endocrinol 2020;11:1-23.
- Fiddes JC, Goodman HM. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet 1981;1:3-18. [PubMed]
- Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev 2010;31:702-55. [Crossref] [PubMed]
- Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med 1994;331:1072-8. [Crossref] [PubMed]
- Anselmo J, Cao D, Karrison T, et al. Fetal loss associated with excess thyroid hormone exposure. JAMA 2004;292:691-5. [Crossref] [PubMed]
- Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol 2010;6:1771-9. [Crossref] [PubMed]
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, et al. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid 2011;21:125-34. [Crossref] [PubMed]
- Khaled H, Al Lahloubi N, Rashad N. A review on thyroid cancer during pregnancy: multitasking is required. J Adv Res 2016;7:565-70. [Crossref] [PubMed]
- Mannathazhathu AS, George PS, Sudhakaran S, et al. Reproductive factors and thyroid cancer risk: meta-analysis. Head Neck 2019;41:4199-208. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013;2013:965212. [Crossref] [PubMed]
- Hatch M, Cardis M. Somatic health effects of chernobyl: 30 years on. Eur J Epidemiol 2017;32:1047-54. [Crossref] [PubMed]
- Glattre E, Kravdal O. Male and female parity and risk of thyroid cancer. Int J Cancer 1994;58:616-7. [Crossref] [PubMed]
- Kravdal O, Glattre E, Haldorsen T. Positive correlation between parity and incidence of thyroid cancer: new evidence based on complete Norwegian birth cohorts. Int J cancer 1991;49:831-6. [Crossref] [PubMed]
- Horn-Ross PL, Chang ET, Clarke CA, et al. Nativity and papillary thyroid cancer incidence rates among Hispanic women in California. Cancer 2012;118:216-22. [Crossref] [PubMed]
- Mack WJ, Preston-Martin S, Bernstein L, et al. Reproductive and hormonal risk factors for thyroid cancer in Los Angeles County females. Cancer Epidemiol Biomarkers Prev 1999;8:991-7. [PubMed]
- Sakoda LC, Horn-Ross PL. Reproductive and menstrual history and papillary thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol Biomarkers Prev 2002;11:51-7. [PubMed]
- Horn-Ross PL, Canchola AJ, Ma H, et al. Hormonal factors and the risk of papillary thyroid cancer in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev 2011;20:1751-9. [Crossref] [PubMed]
- Struve CW, Haupt S, Ohlen S. Influence of frequency of previous pregnancies on the prevalence of thyroid nodules in women without clinical evidence of thyroid disease. Thyroid 1993;3:7-9. [Crossref] [PubMed]
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol 2014;123:1118-32. [PubMed]
- Kung AWC, Chau MT, Lao TT, et al. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab 2002;87:1010-4. [Crossref] [PubMed]
- Angell TE, Alexander EK. Thyroid nodules and thyroid cancer in the pregnant woman. Endocrinol Metab Clin North Am 2019;48:557-67. [Crossref] [PubMed]
- Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-doppler features. J Clin Endocrinol Metab 2002;87:1941-6. [Crossref] [PubMed]
- Mazzaferri EL. Approach to the pregnant patient with thyroid cancer. J Clin Endocrinol Metab 2011;96:265-72. [Crossref] [PubMed]
- Sullivan SA. Thyroid nodules and thyroid cancer in pregnancy. Clin Obstet Gynecol 2019;62:365-72. [Crossref] [PubMed]
- Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011;21:1081-125. [Crossref] [PubMed]
- Hirsch D, Levy S, Tsvetov G, et al. Impact of pregnancy on outcome and prognosis of survivors of papillary thyroid cancer. Thyroid 2010;20:1179-85. [Crossref] [PubMed]
- Kuy S, Roman SA, Desai R, et al. Outcomes following thyroid and parathyroid surgery in pregnant women. Arch Surg 2009;144:399-406. [Crossref] [PubMed]
- Uruno T, Shibuya H, Kitagawa W, et al. Optimal timing of surgery for differentiated thyroid cancer in pregnant women. World J Surg 2014;38:704-8. [Crossref] [PubMed]
- Rosário PW, Barroso AL, Purisch S. The effect of subsequent pregnancy on patients with thyroid carcinoma apparently free of the disease. Thyroid 2007;17:1175-6. [Crossref] [PubMed]
- Moosa M, Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab 1997;82:2862-6. [Crossref] [PubMed]
- Herzon FS, Morris DM, Segal MN, et al. Coexistent thyroid cancer and pregnancy. Arch Otolaryngol Head Neck Surg 1994;120:1191-3. [Crossref] [PubMed]
- Yasmeen S, Cress R, Romano PS, et al. Thyroid cancer in pregnancy. Int J Gynaecol Obstet 2005;91:15-20. [Crossref] [PubMed]
- Messuti I, Corvisieri S, Bardesono F, et al. Impact of pregnancy on prognosis of differentiated thyroid cancer: clinical and molecular features. Eur J Endocrinol 2014;170:659-66. [Crossref] [PubMed]
- Rakhlin L, Fish S, Tuttle M. Response to therapy status is an excellent predictor of pregnancy-associated structural disease progression in patients previously treated for differentiated thyroid cancer. Thyroid 2017;27:396-401. [Crossref] [PubMed]
- Rakhlin L, Fish S. Pregnancy as a risk factor for thyroid cancer progression. Curr Opin Endocrinol Diabetes Obes 2018;25:326-9. [Crossref] [PubMed]
- Korevaar TIM, Haymart MR. A history of thyroid cancer does not meaningfully complicate pregnancy. Thyroid 2019;29:758-9. [Crossref] [PubMed]
- Spiegel E, Spence A, Czuzoj-Shulman N, et al. Pregnancy outcomes after thyroid cancer. J Perinat Med 2019;47:710-6. [Crossref] [PubMed]
- Pomorski L, Bartos M, Narebski J. Pregnancy following operative and complementary treatment of thyroid cancer. Zentralbl Gynakol 2000;122:383-6. [PubMed]
- Bal C, Kumar A, Tripathi M, et al. High-dose radioiodine treatment for differentiated thyroid carcinoma is not associated with change in female fertility or any genetic risk to the offspring. Int J Radiat Oncol Biol Phys 2005;63:449-55. [Crossref] [PubMed]
- Acıbucu F, Acıbucu DO, Akkar ÖB, et al. Evaluation of ovarian reserve with AMH level in patients with well-differentiated thyroid cancer receiving radioactive iodine ablation treatment. Exp Clin Endocrinol Diabetes 2016;124:593-6. [Crossref] [PubMed]
- Giusti M, Mittica M, Comite P, et al. Anti-Müllerian hormone in pre-menopausal females after ablative radioiodine treatment for differentiated thyroid cancer. Endocrine 2018;60:516-23. [Crossref] [PubMed]
- Mittica M, Dotto A, Comina M, et al. Cross-sectional and prospective study on anti-Müllerian hormone changes in a cohort of pre-menopausal women with a history of differentiated thyroid cancer. Thyroid Res 2020;13:1. [Crossref] [PubMed]
- Yaish I, Azem F, Gutfeld O, et al. A single radioactive iodine treatment has a deleterious effect on ovarian reserve in women with thyroid cancer: results of a prospective pilot study. Thyroid 2018;28:522-27. [Crossref] [PubMed]
- Evranos B, Faki S, Polat S, et al. Effects of radioactive iodine therapy on ovarian reserve: a prospective pilot study. Thyroid 2018;28:1702-7. [Crossref] [PubMed]
Cite this article as: Wojtczak B, Kaliszewski K, Binko M, Sępek M, Mulek R, Rudnicki J, Bolanowski M, Barczyński M. Thyroid oncology in pregnancy. Ann Thyroid 2020;5:14.


