
Regression of papillary thyroid cancer using metformin: a case report
Background
Metformin is a biguanide that has been used for almost a century for the treatment of diabetes (1). Over the past two decades, it has been discovered to have utility and the treatment and prevention of number malignancies. Recently, metformin has also come to the attention of endocrinologists as it has been shown to be associated with a preventive effect on thyroid cancer in population-based studies (2) and to exhibit anti-tumor effects in tissue and animal models (3-11). The standard of care for thyroid cancer is thyroidectomy with radioactive ablative iodine (RAI) in selected patients meeting certain parameters based on recent guidelines (12). To our knowledge, the effect of metformin on thyroid cancer in humans has not been reported before. Herein we present here the first clinical evidence of anti-tumor effect of metformin on papillary thyroid cancer. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aot-18-67).
Case presentation
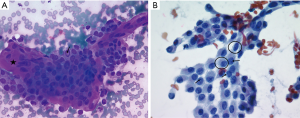
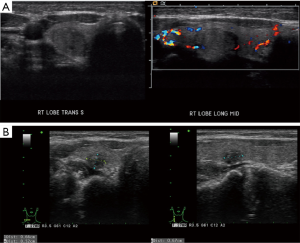
This is a 35-year-old female who noticed a lump in her neck. Her past medical history includes MTHFR with a pulmonary embolus due to her being on oral contraceptives over 10 years prior to presentation without requiring long term need of anticoagulation, but otherwise no significant past surgical or medical history. She did not have any history of neck radiation or any family history thyroid cancer. She was initially evaluated and managed at outside institution. On ultrasound, she was found at that time to have a 1.4×1.1×1.2 cm3 nodule involving the right lobe. This nodule was well circumscribed and hypoechoic. The initial decision was to monitor her without intervention. On a follow-up ultrasound 3 years later, this nodule was found to be 1.5 cm. This nodule was biopsied with FNA at that time showing papillary thyroid carcinoma (Figure 1). On neck ultrasound, she did not have any suspicious central or lateral neck lymphadenopathy. The patient declined recommendations for thyroidectomy and based on the literature search, and she was started on 1,000 mg metformin PO BID, along with a ketogenic diet. She then presented to our endocrine surgery center for a second opinion one year later. She was asymptomatic. TSH, FT3 and FT4 were all found to be within normal limits. On exam, her thyroid was nonpalpable and she did not have lymphadenopathy. Neck ultrasound was repeated which showed the right thyroid nodule to be well circumscribed and hypoechoic but decreased in size to 0.66×0.52×0.67 cm3 (Figure 2), with no evidence of central or lateral neck suspicious lymphadenopathy. At this time, she had been on metformin for a year. She was again recommended to undergo surgery, but the patient wished to be monitored with her ongoing metformin and ketogenic diet. Her FNA slides were reviewed at the Cleveland Clinic as well, agreeing with the diagnosis of papillary thyroid cancer. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Papillary thyroid cancer is one of the most common malignancies seen in the population; currently the 5th most common cancer to afflict women (12). It has also been increasing in incidence, although mortality rates have remained stable at around 1% (12). The traditional treatment plan for this disease involves total thyroidectomy with possible ablation with radioactive I131. Over the past few decades, several studies have demonstrated that this may be overtreatment for lower risk thyroid malignancies. Several low risk thyroid cancers are now being treated with thyroid lobectomy (12). There are some studies monitoring low risk thyroid cancers with routine US surveillance (11).
Metformin was first discovered in 1922 during investigation for treatment of diabetes prior to the widespread availability of insulin. In the 1970s interest was rekindled as a new class of hypoglycemic agents. Over the past two decades, it has been noted that several malignancies have responded or been prevented by metformin, in particular gynecologic, breast, prostate, and colon (13-24). Multiple randomized studies are ongoing to further elucidate the role of metformin in malignancy. This is the first time that papillary thyroid cancer in vivo in humans has been demonstrated to respond to metformin.
In the last 10 years, several basic science papers have demonstrated the possible utility of treatment of thyroid cancer. Several have demonstrated decreased growth and increased apoptosis and necrosis from metformin (3,6). They have also demonstrated potentiation with chemotherapy (10). These apply to all forms of thyroid cancer and not limited to just differentiated papillary and follicular thyroid cancer. Some studies have also demonstrated in vivo studies in mice demonstrating regression and increased survival in mice treated with metformin (10).
The mechanism is likely multifactorial. Metformin is used to treat diabetes by allowing peripheral cells to increase insulin sensitivity in healthy cells and decreases gluconeogenesis. There is evidence that this has a diminished effect of insulin sensitivity in cancerous cells thus decreasing their growth. Several other mechanisms including mTor inhibition, AMPK mediate cell retardation, anoikis mediated cell, cell metabolic derangements, anoikis, and potentiation of chemotherapy and radiation have been demonstrated in vitro (3,5,6,8-10).
There have been only a few papers in the literature looking at metformin on a clinical setting in the thyroid. Cho et al. reported that in the national Korean database, patients who were on metformin were significantly less likely to develop thyroid cancer (2). This finding has been disputed by a similar study looking at the national UK health system (7). One recent paper from Jang et al. found a possible increase in disease free survival in diabetic patients on metformin, however this was a limited finding in a subgroup analysis (4). Decrease in nodule size has been observed in limited studies using metformin. Anil et al. demonstrated a significant but modest decrease in size of thyroid nodules in insulin resistant patients from 12.9±7.6 vs. 11.7±7.2 mm, P<0.0001, however none of these nodules were biopsied in the study (5). A second paper only reached a similar conclusion in nodules <1 cc in size (9). It should be noted that neither of these studies involved any documented cancer.
This study demonstrated biopsy proven cancer with regression. The diagnosis was confirmed by two separate pathologists with review of imaging to confirm regression. Despite this, there are limitations including that this is simply a single case report. Another limitation would include a false positive with the biopsy, however, a thyroid nodule under 1cm would not be an indication for biopsy.
In summary, we report a clinical case related to the effect that metformin may have directly on thyroid cancer clinically. The clinical role needs to be investigated in further studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aot-18-67
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-18-67). Dr. EB reports personal fees from Medtronic, personal fees from Ethicon, personal fees from Integra, personal fees from Aesculap, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sui X, Xu Y, Wang X, et al. Metformin: A Novel but Controversial Drug in Cancer Prevention and Treatment. Mol Pharm 2015;12:3783-91. [Crossref] [PubMed]
- Cho YY, Kang MJ, Kim SK, et al. Protective Effect of Metformin Against Thyroid Cancer Development: A Population-Based Study in Korea. Thyroid 2018;28:864-70. [Crossref] [PubMed]
- Chen G, Xu S, Renko K, et al. Metformin Inhibits Growth of Thyroid Carcinoma Cells, Suppresses Self-Renewal of Derived Cancer Stem Cells, and Potentiates the Effect of Chemotherapeutic Agents. J Clin Endocrinol Metab 2012;97:E510-E520. [Crossref] [PubMed]
- Jang EK, Kim WG, Kwon H, et al. Metformin Is Associated with a Favorable Outcome in Diabetic Patients with Cervical Lymph Node Metastasis of Differentiated Thyroid Cancer. Eur Thyroid J 2015;4:181-8. [Crossref] [PubMed]
- Anil C, Kut A, Atesagaoglu B, et al. Metformin Decreases Thyroid Volume and Nodule Size in Subjects with Insulin Resistance: A Preliminary Study. Med Princ Pract 2016;25:233-6. [Crossref] [PubMed]
- Kheder S, Sisley K, Hadad S, et al. Effects of prolonged exposure to low dose metformin in thyroid cancer cell lines. J Cancer 2017;8:1053-61. [Crossref] [PubMed]
- Becker C, Jick SS, Meier CR, et al. No evidence for a decreased risk of thyroid cancer in association with use of metformin or other antidiabetic drugs: a case-control study. BMC Cancer 2015;15:719. [Crossref] [PubMed]
- Thakur S, Daley B, Gaskins K, et al. Metformin Targets Mitochondrial Glycerophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin Cancer Res 2018;24:4030-43. [Crossref] [PubMed]
- Karimifar M, Aminorroaya A, Amini M, et al. Effect of metformin on thyroid stimulating hormone and thyroid volume in patients with prediabetes: A randomized placebo-controlled clinical trial. J Res Med Sci 2014;19:1019-26. [PubMed]
- Cho SW, Yi KH, Han SK, et al. Therapeutic potential of metformin in papillary thyroid cancer in vitro and in vivo. Molecular and Cellular Endocrinology 2014;393:24-9. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: Areview of active surveillance trial. Eur J Surg Oncol 2018;44:307-15. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- De Souza A, Khawaja KI, Masud F, et al. Metformin and pancreatic cancer: Is there a role? Cancer Chemother Pharmacol 2016;77:235-42. [Crossref] [PubMed]
- Klubo-Gwiezdzinska J, Jensen K, Costello J, et al. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer 2012;19:447-56. [Crossref] [PubMed]
- Ma SJ, Zheng YX, Zhou PC, et al. Metformin use improves survival of diabetic liver cancer patients: systematic review and meta-analysis. Oncotarget 2016;7:66202-11. [Crossref] [PubMed]
- Febbraro T, Lengyel E, Romero IL. Old drug, new trick: Repurposing metformin for gynecologic cancers? Gynecol Oncol 2014;135:614-21. [Crossref] [PubMed]
- Pizzuti L, Vici P, Di Lauro L, et al. Metformin and breast cancer: Basic knowledge in clinical context. Cancer Treatment Reviews 2015;41:441-7. [Crossref] [PubMed]
- Kaewpitoon SJ, Loyd RA, Rujirakul R, et al. Benefits of Metformin Use for Cholangiocarcinoma. Asian Pac J Cancer Prev 2015;16:8079-83. [Crossref] [PubMed]
- Stine JE, Bae-Jump V. Metformin and Gynecologic Cancers. Obstet Gynecol Surv 2014;69:477-89. [Crossref] [PubMed]
- Zhang ZJ, Bi Y, Li S, et al. Reduced Risk of Lung Cancer With Metformin Therapy in Diabetic Patients: A Systematic Review and Meta-Analysis. Am J Epidemiol 2014;180:11-14. [Crossref] [PubMed]
- Rêgo DF, Pavan LM, Elias ST, et al. Effects of metformin on head and neck cancer: A systematic review. Oral Oncol 2015;51:416-22. [Crossref] [PubMed]
- Mei ZB, Zhang ZJ, Liu CY, et al. Survival Benefits of Metformin for Colorectal Cancer Patients with Diabetes: A Systematic Review and Meta-Analysis. PLoS One 2014;9:e91818. [Crossref] [PubMed]
- Hwang IC, Park SM, Shin D, et al. Metformin Association with Lower Prostate Cancer Recurrence in Type 2 Diabetes: a Systematic Review and Meta-analysis. Asian Pac J Cancer Prev 2015;16:595-600. [Crossref] [PubMed]
- Abdelsatir AA, Husain NE, Hassan AT, et al. Potential Benefit of Metformin as Treatment for Colon Cancer: the Evidence so Far. Asian Pac J Cancer Prev 2015;16:8053-8. [Crossref] [PubMed]
Cite this article as: Reznick D, Policarpio-Nicolas ML, Moore EC, Berber E. Regression of papillary thyroid cancer using metformin: a case report. Ann Thyroid 2020;5:27.


