
A case report of severe hypothyroidism in pregnancy complicated by ovarian torsion: the role of medical treatment
Introduction
Overt hypothyroidism affects 1–3 per 1,000 pregnancies in the United States (1). Pregnancy-related complications of overt untreated hypothyroidism can include miscarriage, preeclampsia, placental abruption, preterm birth, low birth weight, and intellectual impairment in the offspring (2). In cases of profound hypothyroidism, bilateral enlargement of the ovaries has been reported. There is a paucity of literature describing pregnant patients with hypothyroidism complicated by ovarian enlargement (3-7).
The published cases thus far have demonstrated resolution of ovarian enlargement with adequate treatment of hypothyroidism. We report, to our knowledge, the first case of ovarian enlargement secondary to severe hypothyroidism in pregnancy complicated by ovarian torsion. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aot-20-59).
Case presentation
A 27-year-old G3P1102 was transferred to our center at 29 3/7 weeks of gestation for further management of acute onset right-sided abdominal pain in the setting of bilateral enlarged ovaries and newly diagnosed hypothyroidism. The patient was diagnosed with hypothyroidism [elevated thyroid stimulating hormone (TSH) 213 µIU/mL (normal 0.5–3.5) and low free thyroxine (FT4) of 0.15 ng/dL (normal 0.9–1.7)] and bilaterally enlarged ovaries at 29 1/7 weeks of gestation upon presenting with abdominal pain to the outside medical center. Levothyroxine was not administered prior to transfer. Upon arrival, the patient was alert and orientated with a weight of 54.9 kg and height of 160 cm (BMI 21.5 kg/m2). The vital signs were within normal limits with blood pressures ranging from 100–110/60–70 mmHg and heart rate of 60–70 beats per minute. The physical exam was significant for right lower quadrant abdominal tenderness with guarding and rebound tenderness. No goiter was palpated. Repeat thyroid labs confirmed an elevated TSH of 154 µIU/mL, low FT4 of 0.36 ng/dL, and an elevated thyroid peroxidase antibody level of 173.0 IU/mL (normal ≤34.0 IU/mL). Upon further questioning, the patient stated that two years prior, she began experiencing symptoms consistent with hypothyroidism including morning facial edema, periorbital edema, voice hoarseness, muscle aches in the hands, constipation, hair loss, and weight gain. The family history was significant for hypothyroidism in her father and sister. She denied personal or family history of ovarian disease. The obstetrical history was notable for two prior cesarean deliveries at 41 and 35 weeks of gestation.
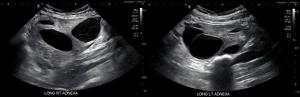
The pelvic ultrasound demonstrated bilaterally enlarged multi-cystic ovaries [right 11.4 cm × 8.3 cm × 8.9 cm (volume 427.5 mL), left 9.3 cm × 5.1 cm × 5.4 cm (volume 133.5 mL)] with no vascular flow on color Doppler within the right ovary concerning for ovarian torsion (Figure 1). The fetal assessment included normal anatomy, an estimated fetal weight of 1,476 g (54th centile by Hadlock), and normal amniotic fluid volume. Endovaginal ultrasound measured a cervical length of 2.9 cm.
A multi-disciplinary meeting was held between the Maternal-Fetal Medicine, Gynecology, and Endocrinology services. The patient was counseled that the etiology of the ovarian enlargement was suspected to be from severe hypothyroidism. She was informed that the ovarian enlargement was expected to reduce once the maternal hypothyroidism was adequately treated. Thus, ovarian cystectomy was not recommended. After considering the pros and cons of management options, the patient elected to proceed with exploratory laparotomy and release of ovarian torsion. Given the severity of hypothyroidism and need for surgery, the patient was given a dose of intravenous levothyroxine 250 µg. A course of betamethasone for fetal maturation was also administered.
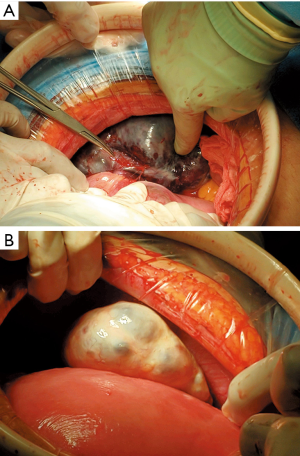
The patient underwent exploratory laparotomy under general anesthesia. The right ovary was dusky with a hemorrhagic appearance and undergoing 1,080° of torsion around the utero-ovarian ligament (Figure 2). The left ovary was enlarged without evidence of torsion. Care was taken to gently untwist the right ovary without rupturing the cysts and replace it within the pelvis. The abdomen was closed in the usual fashion without complications.
On postoperative day #1, the patient received a second dose of intravenous levothyroxine 250 µg followed by a maintenance dose of oral levothyroxine 100 µg daily starting postoperative day #3. The patient developed postoperative ileus and was managed with supportive measures. Her vitals remained within normal limits, and she was discharged on postoperative day #5. At 31 4/7 weeks of gestation, the TSH remained elevated at 97.1 µIU/mL with a normal FT4 of 0.97 ng/dL, thus, the levothyroxine was increased to 150 µg daily.
At 32 3/7 weeks of gestation, repeat ultrasound demonstrated decreasing ovarian size [right 10.6 cm × 6.6 cm × 6.4 cm (volume 234.4 mL), left 5.8 cm × 4.7 cm × 4.6 cm (volume 65.7 mL)]. At 35 4/7 weeks of gestation, TSH was 5.01 µIU/mL and normal FT4 of 1.26 ng/dL. Levothyroxine was further increased to 175 µg daily to reach a target TSH level of less than 2.5 µIU/mL.
At 36 4/7 weeks of gestation, she was diagnosed with severe preeclampsia and underwent a repeat low transverse cesarean delivery. The uterus was unable to be exteriorized due to adhesions and therefore the ovaries were unable to be visualized at that time of delivery. A male neonate was delivered with a birth weight of 2,250 g. The Apgar scores at 1 and 5 minutes were 8 and 9, respectively.
Postpartum, the patient was continued on levothyroxine 175 µg daily. She was doing well at 7 weeks postpartum with decreased muscle cramps and less constipation. Bilateral ovaries appeared normal in size with follicles present on ultrasound [right 4.4 cm × 3.9 cm × 2.6 cm (volume 23.1 mL), left 4.2 cm × 3.0 cm × 3.6 cm (volume 23.2 mL)]. Given the low TSH of 0.15 µIU/mL, the levothyroxine was decreased to 100 µg daily. At last follow-up of 12 months postpartum, the patient was intermittently compliant on levothyroxine 150 µg daily with a TSH of 29.3 µIU/mL and FT4 of 1.34 ng/dL, but otherwise doing well.
The male neonate initially required management for hypoglycemia, but otherwise had an uncomplicated nursery stay. Neonatal thyroid tests were normal on day of life 3 with a TSH of 9.44 µIU/mL (normal <29 µIU/mL). The infant was followed in our Pediatric Clinic and was meeting all developmental milestones as of 12 months of age. Figure 3 outlines the relevant pregnancy and postpartum events.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
We report a case of ovarian enlargement secondary to severe hypothyroidism in pregnancy complicated by ovarian torsion. The patient underwent urgent surgical intervention to relieve the torsion and initiation of levothyroxine to establish a euthyroid state and normalize the bilaterally enlarged ovaries. Although ovarian torsion in the setting of hypothyroidism has been reported in a non-pregnant patient (8), this is the first case to our knowledge describing the complication in pregnancy. The additional strength of our case report is the postnatal follow-up demonstrating normal development of the infant at 12 months of age.
The underlying pathophysiology as to how severe hypothyroidism leads to ovarian enlargement is not entirely understood. TSH receptors are present in ovarian tissue (i.e., surface epithelium, stromal cells, oocytes, granulosa cells) and can be stimulated by recombinant TSH in-vitro (9). These receptors are mediated via a cyclic adenosine monophosphate (cAMP) protein kinase A pathway, and have an increased production of cAMP after being stimulated by TSH (9). Hypothyroidism may also increase collagen deposition leading to ovarian hypertrophy (10) or stromal edema (11). Furthermore, TSH can produce weak follicle stimulating hormone (FSH) activity due to the presence of similar alpha chains, thus producing gonadal stimulation through FSH receptors (12). In our patient, the significantly elevated TSH (>100 µIU/mL) may have activated FSH receptors and contributed to ovarian growth.
Ovarian cyst formation in hypothyroidism is also likely multifactorial and may be due to hypothalamic pituitary dysfunction and alterations in ovarian paracrine and autocrine signaling (11,13,14). In addition, elevated TSH in combination with normal FSH levels may in part activate FSH receptors, leading to profound stimulation of ovarian follicles (14). Histologically, the ovarian cysts are benign with myxedematous infiltration into the ovarian stroma without luteinization of the theca interna (4,11). In our case report, we are limited by the lack of histologic evaluation of the ovarian cysts because neither cystectomy or oophorectomy were performed.
The majority of reported cases of ovarian enlargement secondary to hypothyroidism during pregnancy have resolved after appropriate treatment with levothyroxine within approximately 3 months (3,5-7). Our patient was within this timeframe and had decreasing ovarian volumes 3 weeks after initiating levothyroxine and normal appearing ovaries on ultrasound 14 weeks after starting levothyroxine. Given the severity of hypothyroidism, our patient was given two consecutive doses of intravenous levothyroxine in order to rapidly increase the pool of available thyroid hormone (15,16).
The importance of adherence to levothyroxine should be highlighted. In the setting of inadequate medical management, the cysts may persist or reoccur, which may increase the risk of retorsion. In a study of surgically proven adnexal torsion, there was a 21% risk of adnexal torsion recurrence in patients treated by release of torsion alone compared to 12% who underwent fenestration and 5% who underwent cystectomy (17).
However, the ovarian cysts may not resolve despite levothyroxine therapy. Borna et al. reported a case of a pregnant patient at 20 weeks of gestation who presented with bilaterally enlarged cystic ovaries and hypothyroidism (4). Despite 18 weeks of adequate treatment with levothyroxine, the ultrasound did not demonstrate marked reduction in ovarian size and therefore the patient required ovarian cyst aspiration and a wedge biopsy at the time of cesarean delivery. Ten weeks post-delivery, the ultrasound showed normal sized ovaries (4). Reasons for why ovarian enlargement may persist despite adequate treatment in pregnant patients with bilateral ovarian cysts may be due in part to their elevated levels of human chorionic gonadotropin or mutant FSH receptors (18).
Conclusions
In conclusion, hypothyroidism should be considered in the evaluation of bilaterally enlarged multicystic ovaries even in the setting of advanced pregnancy. In this subset of patients, adequate thyroid hormone replacement not only treats the underlying hypothyroidism, but may avoid unnecessary surgical intervention for the ovarian cysts.
Patient perspective
Although the patient had a difficult pregnancy, she was grateful for the multidisciplinary team approach.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aot-20-59
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-59). Dr. RJP reports personal fees from Ferring Pharmaceuticals, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol 2006;108:1283-92. [Crossref] [PubMed]
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017;27:315-89. [Crossref] [PubMed]
- Cardoso CG, Graca LM, Dias T, et al. Spontaneous ovarian hyperstimulation and primary hypothyroidism with a naturally conceived pregnancy. Obstet Gynecol 1999;93:809-11. [PubMed]
- Borna S, Nasery A. Spontaneous ovarian hyperstimulation in a pregnant woman with hypothyroidism. Fertil Steril 2007;88:705.e1-3. [Crossref] [PubMed]
- Zhou YD, Teng YC, Jiang RZ, et al. A case of a naturally conceived pregnancy associated with multiple ovarian cysts in a patient with severe untreated primary hypothyroidism. Gynecol Endocrinol 2012;28:686-7. [Crossref] [PubMed]
- Nappi RG, Di Naro E, D'Aries AP, et al. Natural pregnancy in hypothyroid woman complicated by spontaneous ovarian hyperstimulation syndrome. Am J Obstet Gynecol 1998;178:610-1. [Crossref] [PubMed]
- Chaverri AP, Solis BEA, Paulin FD, et al. Hyperreactio luteinalis and hypothyroidism: A case report. Case Rep Womens Health 2018;21:e00094 [Crossref] [PubMed]
- Van Voorhis BJ, Neff TW, Syrop CH, et al. Primary hypothyroidism associated with multicystic ovaries and ovarian torsion in an adult. Obstet Gynecol 1994;83:885-7. [PubMed]
- Aghajanova L, Lindeberg M, Carlsson IB, et al. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online 2009;18:337-47. [Crossref] [PubMed]
- Adams WC, Leathem JH. Influence of Hypothyroidism and Chorionic Gonadotrophin on Ovarian Collagen in the Rat. Endocrinology 1964;75:138-9. [Crossref] [PubMed]
- Hansen KA, Tho SP, Hanly M, et al. Massive ovarian enlargement in primary hypothyroidism. Fertil Steril 1997;67:169-71. [Crossref] [PubMed]
- Anasti JN, Flack MR, Froehlich J, et al. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab 1995;80:276-9. [PubMed]
- Fishman J, Hellman L, Zumoff B, et al. Influence of thyroid hormone on estrogen metabolism in man. J Clin Endocrinol Metab 1962;22:389-92. [Crossref] [PubMed]
- Muderris II, Boztosun A, Oner G, et al. Effect of thyroid hormone replacement therapy on ovarian volume and androgen hormones in patients with untreated primary hypothyroidism. Ann Saudi Med 2011;31:145-51. [Crossref] [PubMed]
- Nicoloff JT, LoPresti JS. Myxedema coma. A form of decompensated hypothyroidism. Endocrinol Metab Clin North Am 1993;22:279-90. [Crossref] [PubMed]
- Holvey DN, Goodner CJ, Nicoloff JT, et al. Treatment of Myxedema Coma with Intravenous Thyroxine. Arch Intern Med 1964;113:89-96. [Crossref] [PubMed]
- Tsafrir Z, Hasson J, Levin I, et al. Adnexal torsion: cystectomy and ovarian fixation are equally important in preventing recurrence. Eur J Obstet Gynecol Reprod Biol 2012;162:203-5. [Crossref] [PubMed]
- De Leener A, Montanelli L, Van Durme J, et al. Presence and absence of follicle-stimulating hormone receptor mutations provide some insights into spontaneous ovarian hyperstimulation syndrome physiopathology. J Clin Endocrinol Metab 2006;91:555-62. [Crossref] [PubMed]
Cite this article as: Thomas KC, Chon AH, Mestman J, Nguyen CT, Paulson RJ, Johnson B. A case report of severe hypothyroidism in pregnancy complicated by ovarian torsion: the role of medical treatment. Ann Thyroid 2021;6:6.



