
All roads lead to the thyroid gland: review of two popular “scarless” thyroidectomy approaches
Introduction
Thyroidectomy is the most common procedure performed in endocrine surgery. Thyroid surgery has evolved dramatically from its original inception in 1906 by Theodore Kocher. The last 20 years have shown an increased trend towards remote access endoscopic and, more recently, robotic techniques (1). This is driven by evidence which shows that quality of life in post thyroidectomy patients is significantly affected by the mere presence of a visible cervical scar (2,3).
A variety of different remote access approaches have been developed, primarily in Asia where there is great cultural stigma associated with anterior neck scar. These utilize incisions in the axilla, periareolar area of the breast, retroauricular area and vestibular mucosa. Despite great success seen in the East, the adoption has been slower in North America due to various cultural, regulatory, and fiscal system differences. However, as more data emerges showing comparable outcomes to conventional open thyroidectomy, plus increased patient satisfaction and demand, enthusiasm amongst surgeons is growing.
The transoral endoscopic vestibular approach (TOETVA) and the robotic bilateral axillo-breast approach (BABA) are the two most common remote access approaches to date. This article will review these two techniques, offering a comparison and highlighting the clinical trend and future implications.
TOETVA
TOETVA is the most commonly performed variation of transoral endoscopic thyroidectomy. Although some consider TOETVA as a “natural orifice transluminal endoscopic surgery,” the approach doesn’t involve the oral cavity nor the luminal section of the aerodigestive system. The current TOETVA technique was first described in a cadaveric series by Richmon in 2011 (4). Six years later, Anuwong in Bangkok, Thailand published the first human large series of 60 patients (5). Since that initial sentinel publication in 2016 there has been a growing interest in the technique and increasing efforts to establish it here in the US. Chronologically, TOETVA is the last of the remote access techniques to emerge but, unlike the majority of its predecessors, the general consensus seems to be, as Dr. Yeh put it in a JAMA Surg commentary article to Dr. Anuwong’s initial data, that this one “just might have legs” (6).
The basic principles of the TOETVA operation, when taken at face value, certainly seem more feasible than other described techniques which entail use of the robotic platform or require extensive flap dissection to approach the thyroid from an unfamiliar and oftentimes unnatural angle. Here, the flap dissection plane is similar to that of open thyroidectomy and a basic laparoscopic set up is employed. The reported learning curve of 7–11 cases is not overwhelming (7-9). Finally, and perhaps most convincing of all, is that fact that this technique alone, with its use of 3 rapidly healing inner lip mucosal incisions, is truly scarless.
The adaptation of this technique in North America has been relatively steady but is not yet widely disseminated. The first consecutive series of cases performed in the US was reported by Udelsman et al. at Yale University in 2016 followed by Inabnet et al. at Mount Sinai Beth Israel (10,11). Since then, a growing body of experience has more firmly established a comparable safety profile to that of open thyroidectomy for appropriately selected patients, with few drawbacks other than increased operative time (12). Many institutional and surgical society sponsored workshops have been attempted to aid in the promotion of the technique.
BABA
The BABA endoscopic thyroidectomy, developed in 2004 at Seoul National University Hospital in South Korea, is a remote access technique that uses 4 small, widely spaced incisions to provide ideal triangulation of instruments with a familiar midline view of the thyroid (13) (Figure 1). While initially developed using standard laparoscopy, the technique quickly adapted a robotic platform keeping in trend with the high-volume scarless thyroid centers in Asia (14). This addition allowed for greater dexterity and surgeon autonomy, given the use of the fourth arm, in a challenging working space.
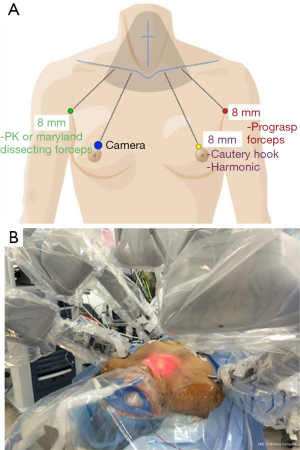
While the BABA thyroidectomy is well established in Asia, it remains rare here in the United States. Despite preceding Anuwong’s TOETVA, the robotic BABA has been slower to find its place in North America, likely due to the robotic platform and the relatively steeper learning curve of 35–40 cases that accompanies this technology (9,14). Nevertheless, the safety profile has been well-established in Asia and the first series of 142 cases performed in the United States was recently submitted for publication. This outcomes based study shows that the robotic BABA technique is applicable to the US patient population with similarly excellent results and low rate of both standard and technique-specific complications (unpublished institutional data).
After the challenges encountered with the robotic transaxillary thyroidectomy (RTT), many experienced endocrine surgeons seem to be wary of robotic thyroidectomies and its progress is further hampered by the fact that there are no U.S. Food and Drug Administration (FDA) approved robotic devices at this time. However, the BABA technique, with its familiar midline view and widely spaced incisions, is more compatible with the robotic platform than that of the crowded unilateral trocar positioning required by the robotic transaxillary approach and is more adaptable for various benign and malignant thyroid disease. Its cosmetic outcome is excellent with high patient satisfaction reported (13,15). While it cannot technically be called “scarless,” when healed the 4 strategically placed incisions are nearly invisible. Furthermore, these incisions heal well even in those patients with a history of keloids or hypertrophic scars.
Surgical indications, contraindications and outcomes
Patient selection
A vital step when attempting to implement a novel surgical technique is that of inclusion criteria and patient candidacy. There is some variability amongst authors but the inclusion criteria for TOETVA are relatively well-established with little variation since Anuwong’s 2016 report of the first 60 human cases. These include the following in a thyroid gland ≤10 cm in size: (I) FNA-proven benign and indeterminate thyroid nodules <6 cm, (II) benign disease (goiter, toxic nodule), (III) well-controlled Grave’s disease, (IV) well-differentiated T1 thyroid carcinoma (≤2 cm) without evidence of extrathyroidal extension or central/lateral compartment spread, and (V) grade I substernal goiters (no extension below the clavicle). Contraindications include previous neck surgery, history of head/neck radiation, chin or mandibular surgery/implants, active oral cavity infections or cancer as well as patients unfit for surgery or who cannot tolerate anesthesia (5,16-18).
The initial indications for endoscopic BABA were similar to those of TOETVA. However, these have expanded with many years of experience and the robotic adaptation. These include all of the TOETVA indications with the addition of well-differentiated carcinoma (DTC) up to 4 cm in size and thyroid nodules up to 8–10 cm in size. Here, preoperative evidence of central neck or lateral neck metastasis is not a contraindication as both areas are easily reached via this approach. Additionally, the first series of cases in North America pushes the boundaries even further by showing the feasibility of robotic BABA even in the face of larger more locally advanced (i.e., strap muscle invasion) primary cancers. Absolute contraindications include large goiters with a significant substernal component, locally invasive cancer cases beyond strap muscle involvement, patients with active breast cancer issues, or females who are actively breastfeeding (19,20).
Outcomes
At this time, both approaches have proven safety profiles comparable to that of open thyroidectomy with minimal approach specific complications (e.g., flap seroma, wound infection) (12,19,21,22). These results are extrapolated primarily from a systematic review by Camenzuli et al. (23). This study evaluated all eligible reports of transoral endoscopic surgery (robotic variation was excluded) from 2011 to 2018 for a cumulative total of 785 patients (23). For the robotic BABA approach these results were taken primarily from the largest single institution series of 1,026 patients by Lee et al. at Seoul National University Hospital in South Korea (19). The reported complication rates from both studies are shown in Table 1. For open thyroidectomy the reported rates of transient and permanent hypoparathyroidism are 6.9–46% and up to 12.1%, respectively while those of transient and permanent RLN palsy are 0.4–12% and 5–6%, respectively (24-26). The incidences of these complications for both novel techniques are well within these ranges. The complication rates are noticeably higher for BABA in all categories listed but several factors should be taken into consideration. In the BABA study 872/1,026 (85%) patients underwent total thyroidectomy with ipsilateral prophylactic central lymph node dissection while only 262/785 (33%) of the patients across the 16 included TEOTVA studies underwent total thyroidectomy with a very small percentage of those undergoing concomitant central neck dissection (19,23). The results from these TOETVA and BABA studies were from a small group of expert thyroid surgeons with extensive experience in these advanced novel techniques and likely cannot be generalized.
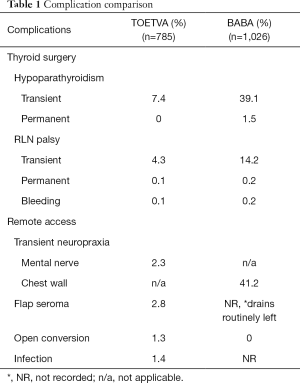
Full table
In any remote-access surgery there is always a chance for an open conversion due to poor visualization, difficulties with hemostasis, or intraoperative complications (Table 1). Although the incidence is low, open conversion should be discussed with patients for optimal safety and outcome. Lee et al. had a 0% open conversion rate in their robotic series (19). However in the initial report of endoscopic BABA from this institution there was a 2.9% incidence of open conversion (13). In the first reported series of 142 cases of robotic BABA in the US, the incidence of open conversion was 2.8%. This is likely attributed to larger average tumor size, higher percentage of Grave’s patients and higher percentage of thyroiditis on final pathology. Additionally, the rates of hypoparathyroidism and RLN palsy were significantly lower in the US study where prophylactic central neck dissections are rarely performed. These rates were 6% and 0.7% for temporary and permanent hypoparathyroidism, respectively and 1.4% and 0.7% for temporary and permanent vocal cord palsy, respectively.
Cosmesis does not come without a price. New approach-specific complications accompany these novel surgical techniques due to the dissection planes necessary to access the thyroid gland (Table 1). For TOETVA, the thyroid is accessed through the oral mucosa and so is considered a clean contaminated surgery. Additionally, the surgeon must be vigilant to avoid thermal injury and tears to the fragile skin of the face and neck as well as oral commissure tears. For BABA, seroma is a risk given the large chest wall flap. Tracheal injury and esophageal perforation have also been reported (27). Both BABA and TOETVA, can have flap related complications including flap skin numbness and paresthesia. With TOETVA, the mental nerve is at risk during trocar placement. This injury produces numbness, tingling and burning of the chin and lips that can persist for several weeks to beyond 6 months. With the modification of trocar placement and a renewed understanding of variation in mental nerve anatomy, incidence and severity of this particular complication have decreased (8,22). Given the relatively more extensive flap dissection required of the BABA approach patients do often experience chest wall neuropraxia in the immediate postoperative period. Retrospectively the Korean group out of Seoul National University Hospital noticed that this seemed to improve after a 3-month period (28,29). The group prospectively studied this, using 3 objective measures of chest was sensation, in a small group of patients and found that the chest wall sensory changes experienced at 1 month post operatively normalized by the 3-month mark (30).
Another rare but feared complication that can be seen with the transoral approach is carbon dioxide air embolism related to initial insufflation in setting of vessel injuries during blunt dissection for the flap. The overall reported incidence of this complication is 0.6% (N=5) (23).
Pros/cons
These two approaches are entirely distinct surgical entities and a direct comparison is not an easy feat. Assessing their advantages and limitations requires a comprehensive understanding of the mechanics of each approach as well as knowledge of the overall goals of remote access surgery (Table 2). In minimally invasive surgery, technical ability is limited by the mechanics of the instruments, surgical platform and the approach design. As shown in Table 2, the only overlap here is a common midline view of the thyroid gland. This is useful as it allows for bilateral surgery, something that is lacking in other approaches such as the transaxillary and retroauricular approaches.
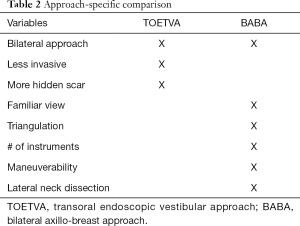
Full table
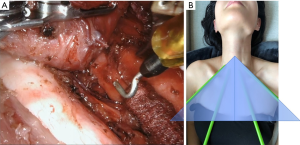
TOETVA’s more limited flap space and dissection plane can be considered less invasive and more in line with the principles of minimally invasive surgery. The tradeoff to this is that trocar placement is limited by the width of the patient’s lips (facial anthropometric dimensions). Such spatial limitation results in a narrow parallel approach angle and less than ideal instrument triangulation and camera visibility (Figure 2). These limitations are augmented by the use of the laparoscopic instruments which are fulcrum and leverage based leading to instrument collisions. Furthermore, despite the midline view of the thyroid, TOETVA dissection proceeds in an unfamiliar cranial to caudal manner with the recurrent laryngeal nerve identified and traced from its insertion at the cricothyroid muscle (Figure 3). However, most endocrine surgeons are facile with laparoscopic instruments which helps with the learning curve and maneuverability after certain adjustments.
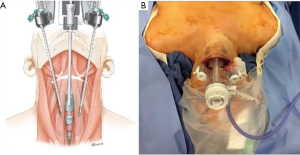
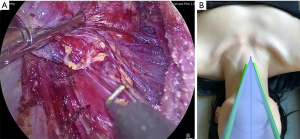
Similar to BABA, the robotic platform has recently been applied to the transoral technique. The transoral robotic thyroidectomy (TORT) is a relatively new application, the first case series of 4 patients was reported in 2015, but it is gaining in popularity largely driven by Dr. Hoon Yub Kim of South Korea (31,32). The articulating instruments are particularly advantageous given the spatial limitations of trocar placement, although a fourth trocar is introduced in the axilla (33).
BABA requires a larger and more time-consuming flap creation, but the payoff is an increased working space which allows for an additional fourth working arm, ideal triangulation of instruments. The midline view here is more familiar to that of conventional open surgery allowing for a nearly identical dissection, including nerve identification and tracing (27) (Figure 4). The use of the robotic platform does come with an increased cost, longer operating time, need for trained robotic OR team, and steeper learning curve, but the articulating instruments and superior views allow for an operative approach that more closely mimics the finesse of an open thyroidectomy.
Robotic BABA expands the application of remote access thyroidectomy for cancer patients. The larger flap and wider working space coupled with the robot’s articulating instruments make endoscopic central and lateral neck dissections feasible, something that is made much harder with TOETVA’s laparoscopic instruments and narrow working space (34-37). Several studies have demonstrated the efficacy of robotic modified radical neck dissections using the BABA approach in patients with advanced papillary thyroid cancer with lateral neck spread, including bilateral neck involvement. All demonstrated outcomes comparable to open neck dissection (35-37). Additionally, BABA’s more forgiving axillary fold extraction site allows for removal of larger cancer specimens without risk of capsular disruption (20). The role of TOETVA for thyroid cancer beyond papillary microcarcinoma is still unclear (38). At present, the technique is recommended for DTC <2 cm in size without clinically positive nodes or extrathyroidal extension (18,39). A major limitation here is the vestibular extraction site. The presence of a rigid mental protuberance, mentalis muscle and the small vestibular mucosal incisions limits the specimen removal for tumors larger than 2 cm (16,40). A variation of the TORT developed by Dr. Kim of South Korea places the fourth arm in the right axilla to be used for countertraction during the case. This can then be used as the extraction site, which is especially useful for larger specimens (33). The midline approach does lend itself to central neck dissection and this has been described in the literature. Yet, its application at this time is limited to prophylactic central neck dissection, a practice that is largely going to the wayside with the more conservative 2015 ATA guidelines, and intraoperatively discovered nodal disease (16,41). A recent paper by Grogan et al. examined what percentage of US thyroidectomy patients would be eligible for TOETVA, based on standardized inclusion criteria. The indications were benign disease, indeterminate cytology and cancer. The latter had the lowest percentage eligibility at 29% while 69% and 76% of benign disease and indeterminate nodules, respectively, were eligible (42).
When evaluating oncological safety of thyroidectomy, serum thyroglobulin (Tg) level and radioiodine uptake on whole body scan are common parameters to assess surgical completeness. Given the high prevalence of small differentiated thyroid cancer in Asia, the use of BABA in DTC has been well-studied by the group from Seoul National Hospital in South Korea. Their data repeatedly show that outcomes are comparable to conventional open surgery for small DTC (19,27,43). In 2017, they published data for larger cancers, 2–4 cm in size, and found equally favorable results (20). For this study, the median follow up period was 40.2 months. TOETVA, as a more recently described technique, is in an earlier state of investigation for malignant cases. Additionally, TOETVA is more often performed for indeterminate nodules, or more recently, small DTC in low risk patients for which lobectomy, rather than total thyroidectomy, is preferred (44). As TOETVA becomes more popularized worldwide its use is being broadened and efforts to show its oncological safety are emerging. Recently, Ahn and Yi published a series of 150 patients who underwent TOETVA for thyroid cancer, however only 40 patients had total thyroidectomy. Regardless this is the largest series to date of TOETVA for thyroid cancer which evaluates surgical completeness. Both number of central lymph nodes harvested and Tg levels were comparable to open thyroidectomy and the median follow up period was 105 days (45).
Discussion
We have described two of the more popular and well-studied remote access techniques to date. These are two very distinct surgical approaches to thyroidectomy but share the same goal: to safely remove the thyroid with the smallest possible footprint without compromising surgical excellence.
These advanced techniques should be performed by high volume thyroid surgeons (17). An intimate understanding of the central neck anatomy is crucial to safe execution of a remote access approach. A background in laparoscopic surgery and knowledge of the general principles of minimally invasive surgery are also important. BABA can be performed endoscopically but is well-suited to the robotic platform which has greatly advanced the approach. If the surgeon wishes to pursue this technique, robotic credentialing and proficiency are essential. For some, TOETVA may seem a more realistic undertaking given the shorter learning curve and use of standard laparoscopic instruments. Those with a strong robotic background may wish to pursue robotic BABA given its potential for use in more advanced thyroid disease.
These are high specialized techniques and the data reported in this review are from a select few expert high-volume surgeons who have long passed the initial learning curves. Therefore, these favorable results are not generalizable. There is a paucity of data on the results of this approaches by novice surgeons, but one must assume that conversion and complication rates would be higher. For those serious about pursuing these techniques it is of the utmost importance to attain sufficient training and be proctored by a surgeon experienced in this technique for the first several cases.
For those new to remote access thyroidectomy, it is advisable to select one approach and master it rather than trying to learn two distinct, highly specialized techniques simultaneously. Which approach one selects is a matter of preference depending on the individual surgeon, their training experience, and the institutional support system. At this time, there is limited data regarding the use of TOETVA for more advanced malignant pathology while the robotic BABA technique and its large and readily extensible working area lend itself to more advanced pathology, both benign and malignant.
As the experience and the dissemination of these techniques grow over time, disease specific algorithms and indications should be established based on the outcome and innate limitations of each techniques.
At this time, more long-term data is needed to assess overall efficacy, particularly for the treatment of malignant disease. However, regardless of the approach, with appropriate patient selections combined with surgeon’s innovative spirit and determination, these techniques will continue to evolve with excellent surgical outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathon Russell and Jeremy Richmon) for the series “The Management of Thyroid Tumors in 2021 and Beyond” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-47). The series “The Management of Thyroid Tumors in 2021 and Beyond” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sukpanich R, Sanglestsawai S, Seib CD, et al. The influence of cosmetic concerns on patient preferences for approaches to thyroid lobectomy: A discrete choice experiment. Thyroid 2020;30:1306-13. [Crossref] [PubMed]
- Choi Y, Lee JH, Kim YH, et al. Impact of Postthyroidectomy Scar on the Quality of Life of Thyroid Cancer Patients. Ann Dermatol 2014;26:693-9. [Crossref] [PubMed]
- Arora A, Swords C, Garas G, et al. The perception of scar cosmesis following thyroid and parathyroid surgery: A prospective cohort study. Int J Surg 2016;25:38-43. [Crossref] [PubMed]
- Richmon JD, Pattani KM, Benhidjeb T, et al. Transoral robotic-assisted thyroidectomy: A preclinical feasibility study in 2 cadavers. Head Neck 2011;33:330-3. [PubMed]
- Anuwong A. Transoral Endoscopic Thyroidectomy Vestibular Approach: A Series of the First 60 Human Cases. World J Surg 2016;40:491-7. [Crossref] [PubMed]
- Yeh MW. Thyroid Surgery Through the Mouth Might Not Be as Crazy as It Sounds. JAMA Surg 2018;153:28. [Crossref] [PubMed]
- Razavi CR, Vasiliou E, Tufano RP, et al. Learning Curve for Transoral Endoscopic Thyroid Lobectomy. Otolaryngol Head Neck Surg 2018;159:625-9. [Crossref] [PubMed]
- Anuwong A, Ketwong K, Jitpratoom P, et al. Safety and Outcomes of the Transoral Endoscopic Thyroidectomy Vestibular Approach. JAMA Surg 2018;153:21-7. [Crossref] [PubMed]
- Russell JO, Razavi CR, Al Khadem MG, et al. Anterior cervical incision‐sparing thyroidectomy: Comparing retroauricular and transoral approaches. Laryngoscope Investig Otolaryngol 2018;3:409-14. [Crossref] [PubMed]
- Udelsman R, Anuwong A, Oprea AD, et al. Trans-oral Vestibular Endocrine Surgery: A New Technique in the United States. Ann Surg 2016;264:e13 [Crossref] [PubMed]
- Inabnet WB, Suh H, Fernandez-Ranvier G. Transoral endoscopic thyroidectomy vestibular approach with intraoperative nerve monitoring. Surg Endosc 2017;31:3030. [Crossref] [PubMed]
- Shan L, Liu J. A Systemic Review of Transoral Thyroidectomy. Surg Laparosc Endosc Percutan Tech 2018;28:135-8. [Crossref] [PubMed]
- Choe JH, Kim SW, Chung KW, et al. Endoscopic Thyroidectomy Using a New Bilateral Axillo-Breast Approach. World J Surg 2007;31:601-6. [Crossref] [PubMed]
- Liu SYW, Kim JS. Bilateral axillo-breast approach robotic thyroidectomy: review of evidences. Gland Surg 2017;6:250-7. [Crossref] [PubMed]
- Koo DH, Kim D, Choi J, et al. In-Depth Survey of Scarring and Distress in Patients Undergoing Bilateral Axillo-Breast Approach Robotic Thyroidectomy or Conventional Open Thyroidectomy. Surg Laparosc Endosc Percutan Tech 2015;25:436-9. [Crossref] [PubMed]
- Anuwong A, Sasanakietkul T, Jitpratoom P, et al. Transoral endoscopic thyroidectomy vestibular approach (TOETVA): indications, techniques and results. Surg Endosc 2018;32:456-65. [Crossref] [PubMed]
- Razavi CR, Tufano RP, Russell JO. Starting a Transoral Thyroid and Parathyroid Surgery Program. Curr Otorhinolaryngol Rep 2019;7:204-8. [Crossref] [PubMed]
- Dionigi G, Lavazza M, Wu CW, et al. Transoral thyroidectomy: why is it needed? Gland Surg 2017;6:272-6. [Crossref] [PubMed]
- Lee KE, Kim E, Koo DH, et al. Robotic thyroidectomy by bilateral axillo-breast approach: review of 1026 cases and surgical completeness. Surg Endosc 2013;27:2955-62. [Crossref] [PubMed]
- Chai YJ, Suh H, Woo JW, et al. Surgical safety and oncological completeness of robotic thyroidectomy for thyroid carcinoma larger than 2 cm. Surg Endosc 2017;31:1235-40. [Crossref] [PubMed]
- Shan L, Liu J. Meta-analysis Comparison of Bilateral Axillo-Breast Approach Robotic Thyroidectomy and Conventional Thyroidectomy. Surg Innov 2019;26:112-23. [Crossref] [PubMed]
- Fernandez-Ranvier G, Meknat A, Guevara DE, et al. Transoral Endoscopic Thyroidectomy Vestibular Approach. JSLS 2019;23:e2019.00036.
- Camenzuli C, Schembri-Wismayer P, Agius J. Transoral Endoscopic Thyroidectomy: A Systematic Review of the Practice So Far. JSLS 2018;22:e2018.00026.
- Thomusch O, Machens A, Sekulla C, et al. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003;133:180-5. [Crossref] [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Cavicchi O, Burgio L, Cioccoloni E, et al. Intraoperative intermittent neuromonitoring of inferior laryngeal nerve and staged thyroidectomy: our experience. Endocrine 2018;62:560-5. [Crossref] [PubMed]
- Choi JY, Lee KE, Chung KW, et al. Endoscopic thyroidectomy via bilateral axillo-breast approach (BABA): review of 512 cases in a single institute. Surg Endosc 2012;26:948-55. [Crossref] [PubMed]
- Lee J, Nah KY, Kim RM, et al. Differences in postoperative outcomes, function, and cosmesis: open versus robotic thyroidectomy. Surg Endosc 2010;24:3186-94. [Crossref] [PubMed]
- Kim SJ, Lee KE, Myong JP, et al. Recovery of sensation in the anterior chest area after bilateral axillo-breast approach endoscopic/robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech 2011;21:366-71. [Crossref] [PubMed]
- Kim SJ, Lee KE, Myong JP, et al. Prospective Study of Sensation in Anterior Chest Areas Before and After a Bilateral Axillo-breast Approach for Endoscopic/Robotic Thyroid Surgery. World J Surg 2013;37:1147-53. [Crossref] [PubMed]
- Lee HY, You JY, Woo SU, et al. Transoral periosteal thyroidectomy: cadaver to human. Surg Endosc 2015;29:898-904. [Crossref] [PubMed]
- Kim HK, Park D, Kim HY. Robotic transoral thyroidectomy for papillary thyroid carcinoma. Ann Surg Treat Res 2019;96:266-8. [Crossref] [PubMed]
- Kim HY, Chai YJ, Dionigi G, et al. Transoral robotic thyroidectomy: lessons learned from an initial consecutive series of 24 patients. Surg Endosc 2018;32:688-94. [Crossref] [PubMed]
- Lee KE, Rao J, Youn YK. Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech 2009;19:e71-5. [Crossref] [PubMed]
- Choi JY, Kang KH. Robotic modified radical neck dissection with bilateral axillo-breast approach. Gland Surg 2017;6:243-9. [Crossref] [PubMed]
- Song RY, Sohn HJ, Paek SH, et al. The First Report of Robotic Bilateral Modified Radical Neck Dissection Through the Bilateral Axillo-breast Approach for Papillary Thyroid Carcinoma With Bilateral Lateral Neck Metastasis. Surg Laparosc Endosc Percutan Tech 2020;30:e18-e22. [Crossref] [PubMed]
- Yu HW, Chai YJ, Kim S, et al. Robotic-assisted modified radical neck dissection using a bilateral axillo-breast approach (robotic BABA MRND) for papillary thyroid carcinoma with lateral lymph node metastasis. Surg Endosc 2018;32:2322-7. [Crossref] [PubMed]
- Luna-Ortiz K, Gómez-Pedraza A, Anuwong A. Lessons Learned from the Transoral Endoscopic Thyroidectomy with Vestibular Approach (TOETVA) for the Treatment of Thyroid Carcinoma. Ann Surg Oncol 2020;27:1356-60. [Crossref] [PubMed]
- Wu YJ, Chi SY, Elsarawy A, et al. What is the Appropriate Nodular Diameter in Thyroid Cancer for Extraction by Transoral Endoscopic Thyroidectomy Vestibular Approach Without Breaking the Specimens? A Surgicopathologic Study. Surg Laparosc Endosc Percutan Tech 2018;28:390-3. [Crossref] [PubMed]
- Chen Y, Chomsky-Higgins K, Nwaogu I, et al. Hidden in Plain Sight: Transoral and Submental Thyroidectomy as a Compelling Alternative to “Scarless” Thyroidectomy. J Laparoendosc Adv Surg Tech A 2018;28:1374-7. [Crossref] [PubMed]
- Razavi CR, Fondong A, Tufano RP, et al. Central neck dissection via the transoral approach. Ann Thyroid 2017;2:11. [Crossref] [PubMed]
- Grogan RH, Suh I, Chomsky-Higgins K, et al. Patient Eligibility for Transoral Endocrine Surgery Procedures in the United States. JAMA Netw Open 2019;2:e194829 [Crossref] [PubMed]
- Lee KE, Koo DH, Kim S, et al. Outcomes of 109 patients with papillary thyroid carcinoma who underwent robotic total thyroidectomy with central node dissection via the bilateral axillo-breast approach. Surgery 2010;148:1207-13. [Crossref] [PubMed]
- Jongekkasit I, Jitpratoom P, Sasanakietkul T, et al. Transoral Endoscopic Thyroidectomy for Thyroid Cancer. Endocrinol Metab Clin North Am 2019;48:165-80. [Crossref] [PubMed]
- Ahn JH, Yi JW. Transoral endoscopic thyroidectomy for thyroid carcinoma: outcomes and surgical completeness in 150 single-surgeon cases. Surg Endosc 2020;34:861-7. [Crossref] [PubMed]
Cite this article as: Mencio M, Fernandez-Ranvier G, Inabnet WB, Suh H. All roads lead to the thyroid gland: review of two popular “scarless” thyroidectomy approaches. Ann Thyroid 2021;6:10.


