
A narrative review of molecular testing for indeterminate thyroid nodules: living with the results
Introduction
Thyroid nodules are prevalent in the United States: by age 50, up to 50–60% of the population have one or more thyroid nodules incidentally found on imaging performed for an unrelated indication (1). Surveillance, Epidemiology, and End Results (SEER) data from 2015–2019 shows thyroid cancer to be the fifth most common malignancy amongst women in the United States, and is associated with excellent outcomes including a >98% 5-year survival (2). Despite its excellent prognosis, patients disproportionately report perceived impairment in quality of life (QOL) compared with more lethal cancers (3).
Thyroid nodules may be biopsied via fine-needle-aspiration (FNA), and the cytological reporting of these results was standardized by the Bethesda classification system in 2009 and updated in 2017 (4,5). With this standardization, nearly 100,000 nodules are graded cytologically indeterminate every year in the US (6). The indeterminate classification includes Bethesda III, or atypia or follicular lesion of undetermined significance, and Bethesda IV, or follicular neoplasm or suspicious for follicular neoplasm. The traditional paradigm for management was either to re-biopsy such a nodule or perform diagnostic surgical resection of the affected lobe of the thyroid. When surgically resected, anywhere from 10–40% of Bethesda III and IV lesions returned malignant on final pathological analysis (5). More recently, molecular testing has emerged as a modality to aid in risk stratifying these indeterminate thyroid nodules to avoid unnecessary diagnostic surgery. Two of the current leading diagnostic tests in this space are the Afirma Gene Sequencing Classifier (GSC) by Veracyte, and ThyroSeq v3 by CBLPath. The initial Afirma test, the Gene Expression Classifier (GEC) utilized microarray measurement of messenger RNA expression of 167 genes. Since then, Afirma has introduced their next generation GSC which seeks to improve the test’s positive predictive value by including analysis of nuclear and mitochondrial RNA gene expression and gene copy number analysis (7). ThyroSeq analyzes both RNA and DNA mutations, with 7 genes assessed in the initial ThyroSeq v0, 13 genes in v1, 56 genes in v2, and finally v3 analyzing 112 genes for mutations, insertions and deletions, fusions, expression alterations, and copy number alterations (8).
Value-based care and patient reported outcomes
In 2022, we are more conscious than ever about optimizing the value of healthcare delivered to patients, which relies on improving quality and minimizing cost. The Afirma GSC and ThyroSeq v3 molecular tests are well validated and can improve the quality of care for patients with thyroid nodules by allowing patients with benign or negative molecular testing to avoid diagnostic surgery. A randomized controlled trial in 2021 by Livhits et al. compared Afirma GSC to ThyroSeq v3 and found a 100% and 97% sensitivity, and 80% and 85% specificity, respectively (9). The outcome of unnecessary surgery avoided was greatly improved with both tests—49% of patients were able to avoid an unnecessary surgery on the basis of a benign or negative molecular test. The other half of the value proposition is cost. Nicholson et al. performed a cost-effectiveness analysis comparing molecular testing with diagnostic lobectomy in the US. The authors concluded that the average expected cost of a molecular testing strategy, including surgery if needed and associated complications, was approximately $14,000 for ThyroSeq v3 and $18,000 for Afirma GSC. The expected cost of proceeding directly to diagnostic lobectomy, including associated complications, was $38,000 (10). In the modern era of shared decision making, physicians not only need to understand outcomes and costs associated with treatment options, but also need to understand how patients view risk, and how their QOL may be affected by different treatment options.
To date, only a few studies have investigated QOL associated with molecular testing for indeterminate thyroid nodules. Our aim in this review is to discuss the findings, implications, and limitations of these studies. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aot.amegroups.com/article/view/10.21037/aot-22-13/rc).
Methods
A literature review was performed using PubMed to search all scientific articles published in the English language over the last 30 years. The search terms used included: “thyroid nodules”, “indeterminate”, “molecular testing” and “quality of life”. We present the detailed search strategy in Table 1. Original papers were included and duplicates, review articles, and those that did not have a meaningful assessment of QOL or molecular testing were removed.
Table 1
| Items | Specification |
|---|---|
| Date of search | August 22, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “thyroid nodules” [Title/Abstract] AND “indeterminate” [Title/Abstract] AND “molecular testing” [Title/Abstract] AND “quality of life” [Title/Abstract] |
| Timeframe | 1990–2022 |
| Inclusion and exclusion criteria | Focus was placed on original papers in English about quality of life observations for patients undergoing molecular testing for indeterminate thyroid nodules; it excluded review articles, duplicates, and those that had no information about quality of life or molecular testing |
| Selection process | Selection was conducted by Vivek Sant and confirmed by Masha Livhits |
Search results and discussions
Of 6 papers resulted by the search strategy, the following were excluded: 1 duplicate, 1 editorial reply, 1 review article, and 1 article without meaningful assessment of QOL. This resulted in 2 original papers which will be discussed further, and through further analysis of references from these papers, we additionally discuss a few pieces of supporting literature. In 2020, Wong et al. published a cross-sectional study of 332 patients at a single institution undergoing FNA of their thyroid nodule (11). The 58 patients with indeterminate cytology on FNA were block randomized by month to undergo either Afirma GEC or ThyroSeq v2. After patients received the cytology and molecular testing results, they completed the ThyPro-39 Short Questionnaire, a validated patient-reported outcome tool for thyroid conditions (12). Four QOL categories relevant to thyroid nodule and cancer were assessed: symptoms of goiter, anxiety, depression, and impaired daily life. Patients answered each question with a score from 0–4, and QOL scores were calculated for each category scaled from 0–100, such that a higher score represented worse QOL.
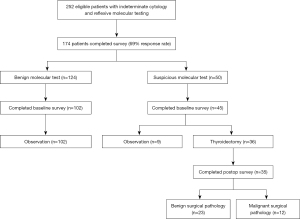
A follow-up study was published by Schumm et al. in 2021 in Annals of Surgical Oncology, and was a longitudinal study following the changes in QOL for patients with indeterminate cytology (13). Following the previous patient population over the next 3 years, 252 patients with indeterminate cytology were block randomized by month to Afirma GSC or ThyroSeq v3. Depending on the results and patient/physician preference, these patients either continued to surgery and subsequent post-operative follow-up or underwent ultrasound surveillance of their nodules. Over 2 years of follow-up, the ThyPro-39 Short Questionnaire was administered at 3 distinct time points: baseline (0–4 months after FNA), early (4–12 months after FNA), and late (12–24 months after FNA). One hundred and seventy-four patients completed surveys and were included for analysis, and the patient flow diagram is presented (Figure 1). The same four QOL categories relevant to thyroid nodule and cancer were assessed as the earlier cross-sectional study, as well as emotional susceptibility, impaired social life, and appearance. Both studies controlled for age, gender, thyroid stimulating hormone (TSH) level and nodule size.
Benign molecular test result compared to benign cytology
The cross-sectional study found that QOL scores were no different in patients with cytologically benign nodules and those with indeterminate nodules with benign molecular testing. The results were consistent on all four axes including goiter, anxiety, depression, and impaired daily life. The longitudinal study found no significant changes in all seven axes (adding emotional susceptibility, impaired social life, and appearance) from baseline through 18 months of ultrasound surveillance, including both early and late follow up. These findings suggest that patients, presumably after appropriate counseling by their physicians, are appropriately reassured that the risk of malignancy associated with a benign molecular test result is very low. Patients do not suffer from anxiety or concern related to the initial indeterminate FNA result, both at the time of initial benign molecular test or during long-term follow-up. This is consistent with the well-validated high sensitivity and negative predictive value of a benign molecular test result with either Afirma GSC or ThyroSeq v3 (9,14,15).
Suspicious molecular test result compared to malignant cytology
Patients with malignant cytology in the cross-sectional study were compared to those with indeterminate cytology and a suspicious molecular test. Malignant cytology was associated with greater impairment of daily life score (25.8±28.3 vs. 8.3±16.6, P=0.003) but no differences in symptoms of goiter, anxiety, or depression. The risk of malignancy associated with a suspicious molecular test result is approximately 40–60%, with patients in either group typically proceeding to surgery for treatment or diagnosis. Given that both groups typically proceed to surgery, it is hard to explain differences in impairment of daily life scores by a difference in expected treatment, unless a significant number of patients with confirmed malignancy are presenting at an advanced cancer stage and are expecting adjuvant treatments such as radioactive iodine or external beam radiation. However, such a potential difference was not explored in the paper. Greater impaired daily life score in confirmed malignancy may alternatively correlate with more anxiety over the known diagnosis of cancer, but this study found no differences in anxiety score. Although a low probability, the above difference may of course exist by chance, or there is perhaps an alternate cause of this greater impairment of daily life that we do not yet completely understand. When considering active surveillance of thyroid cancer and the possibility of expansion of active surveillance to molecular suspicious nodules in the future, it will therefore be important to measure QOL in both subsets, rather than simply assuming they will be the same for both cytological malignancy and molecular suspicious tests. Further analysis of QOL in active surveillance is discussed later in this review.
Suspicious molecular test result compared to benign molecular test result
Not surprisingly, both studies found a suspicious molecular test result to be associated with worse QOL compared to a benign molecular test result at the initial follow-up. The cross-sectional study noted fewer symptoms of goiter (10.4±12.9 vs. 20.5±20.8, P=0.033) and depression (21.0±14.9 vs. 33.3±24.7, P=0.026) in patients with molecular benign test results compared to those with molecular suspicious test results, although there were no differences in symptoms of anxiety or impaired daily life. The longitudinal study reported worse symptoms of goiter, anxiety, and depression at baseline for patients with a suspicious molecular test result. However, a larger proportion of these patients had nodule size >2 cm, which may partially explain worsened symptoms of goiter. Overall, there were no QOL differences between the two groups at early follow-up. The suspicious molecular group that was compared included both patients who subsequently opted to proceed with surgery and those who underwent observation. The lack of sustained difference in QOL between groups likely reflects the large proportion of patients with suspicious molecular testing who underwent surgery and subsequently reported improvement in nearly all QOL domains compared to their baseline.
Suspicious molecular test result—preoperative compared to postoperative QOL scores
The longitudinal study analyzed patients with suspicious molecular test results who underwent surgery and compared QOL pre- and post-operatively. The comparison interval was from baseline to a median of 8 months after surgery. After surgery, patients overall reported significantly improved symptoms of goiter (21.4±23.9 vs. 9.9±10.8, P<0.01), anxiety (35.2±28.0 vs. 23.9±24.0, P=0.049), depression (38.1±23.5 vs. 26.6±18.9, P<0.01), impaired social life (20.4±24.4 vs. 10.9±18.1, P=0.02) and impaired daily life (17.6±22.5 vs. 9.4±15.1, P=0.04). Subgroup analysis was performed for both benign and malignant surgical pathology. Patients with benign surgical pathology reported improved symptoms of goiter (21.4±23.9 vs. 8.3±9.6, P<0.01) and impaired social life (20.4±24.4 vs. 13.9±20.7, P=0.07, trend). Patients with malignant surgical pathology reported improved symptoms of depression (38.1±23.5 vs. 20.4±13.0, P<0.01) and anxiety (35.2±28.0 vs. 19.5±22.4, P=0.07, trend). It is reassuring to note that post-operatively, patients overall noted improvement in nearly all QOL domains, and that the greatest improvement for patients with benign pathology was in symptoms of goiter, while the greatest improvement for patients with malignant surgical pathology was in symptoms of depression. Definitive surgical treatment likely provides patient reassurance of good long-term oncologic outcomes for patients with thyroid cancer.
Suspicious molecular test result with benign surgical pathology compared to benign molecular test result
The longitudinal study compared patients with benign surgical pathology after undergoing surgery for suspicious molecular testing (n=23 with benign surgical pathology out of n=36 undergoing surgery) to patients undergoing observation for benign molecular testing results (n=124). At baseline, no QOL differences were noted. However, at both early and late follow up, depression and appearance were worse in patients who underwent surgery. Such patients, who were ultimately found to have a false positive molecular test, may feel that they have undergone an unnecessary surgery, with the emotional stress, scar, and potential complications that accompany surgery. These results point to the need for continued improvement in the specificity of molecular testing to allow more patients to avoid unnecessary diagnostic surgery.
Suspicious molecular test result—active surveillance
A small subset of patients in the longitudinal study with suspicious molecular testing chose to manage this finding non-operatively with ultrasound surveillance (n=9). When comparing patients with benign and suspicious molecular tests who opted for surveillance, at early follow-up, no differences in QOL were found, apart from improved symptoms of goiter in the patients with suspicious molecular testing [21.1±23.7 (n=45) vs. 11.5±15.5 (n=9), P=0.04]. No statistical tests were performed at late follow up due to the small sample size at that time point. Patients with a suspicious molecular test result opting for active surveillance are likely more worried about the impairment and potential complications from surgery than the risk of potential malignancy. The QOL findings for such patients who self-select to observation may reflect satisfaction with their choice, and this may provide some reassurance to physicians, at least when understanding their patient’s QOL perspective.
These findings track with a 2019 study surveying patients with papillary thyroid microcarcinoma (PTMC), comparing those who underwent active surveillance to those who underwent surgery (16). They found that active surveillance was associated with improved QOL and did not increase fear of disease progression.
What do patients prioritize when choosing a treatment option?
In 2021, Pitt et al. conducted interviews with 85 patients who were diagnosed with indeterminate or malignant thyroid nodules on thyroid nodule FNA (17). A few themes emerged regarding how patients thought about their diagnosis and treatment. Patients frequently expressed (I) fear that the cancer would spread while awaiting surgery, (II) desire to “get it out” (remove the tumor), in an expeditious manner, and belief that this would resolve their issue, (III) strong emotional response to the term “cancer” or even potential cancer (indeterminate cytology) that influenced their treatment choices, and (IV) minimization of the likelihood and severity of surgical risk. These themes help us further understand how patients think about and experience QOL differences based on their treatment choices. While the majority of patients prioritize removal of their cancer, there is clearly a subset of patients who value avoiding surgery if at all possible. Their reasons generally include concerns about the risks of thyroid surgery (in particular, voice change), cosmetic appearance of the scar, and how the possible need for thyroid hormone supplementation may impact QOL. Molecular testing can help to inform the risk of malignancy and potentially separate indolent from more aggressive thyroid cancers, allowing for increasingly individualized patient care based on the expected clinical behavior of the thyroid nodule and the values of the patient. Aligning the treatment plan with patient values whenever possible would likely optimize QOL outcomes.
Limitations
The two primary molecular QOL studies reviewed here have a few limitations. The QOL outcomes of an indeterminate result by itself, without molecular testing, were not assessed, given that all patients reflexively underwent molecular testing. QOL was not assessed prior to FNA biopsy, which would have allowed baseline assessment of QOL for patients with a thyroid nodule. Variables of race, ethnicity, socioeconomic status, and pre-existing co-morbidities including anxiety and depression were not collected, which may affect baseline QOL. The loss of patients during the period of longitudinal follow up may introduce nonresponse bias.
Of note, the cross-sectional study utilized Afirma GEC and ThyroSeq v2, which were the latest available versions of the tests at the time. The follow-on study utilized the newer Afirma GSC and ThyroSeq v3, because they were subsequently made available during the following study period. It has been previously described in the literature that the newer versions of both tests have better diagnostic performance than the older versions (14,18,19). Although this likely doesn’t affect the interpretation of QOL findings between the two papers given their relative concordance, this difference should be noted when considering their relevance to current practice.
The limited number of relevant articles published on this topic and included in this study may limit the conclusions one can draw. The two original articles summarized come from a single academic institution. The applicability of these results outside the US is uncertain, as it has been noted that clinicians in Asia may practice differently than their counterparts in Europe or the US. As noted by Vuong et al. in a meta-analysis, Western countries had a higher resection rate for all Bethesda categories compared to Asian countries, while risk of malignancy in surgically resected specimens was higher for most Bethesda categories in Asian countries compared to Western countries (20). These practice differences coupled with likely cultural differences may make the result of US QOL studies difficult to apply directly to other populations, and thus, further work across geographies, cultures and practice patterns is warranted.
Conclusions
QOL assessments surrounding molecular testing for indeterminate nodules is a relatively nascent field. One of the most reassuring early findings has been that patients view a benign molecular test similarly to benign cytology. A suspicious molecular result may initially be associated with worse QOL compared to benign molecular result, but QOL differences seem to disappear at early follow up, likely reflecting the large proportion of patients with suspicious molecular testing who undergo surgery and subsequently report improvement in nearly all QOL domains compared to their baseline.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vaninder Dhillon and Elizabeth Cottrill) for the series “Improved Quality of Life after Thyroid Surgery” published in Annals of Thyroid. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aot.amegroups.com/article/view/10.21037/aot-22-13/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-22-13/coif). The series “Improved Quality of Life after Thyroid Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 1997;126:226-31. [Crossref] [PubMed]
- Common Cancer Sites - Cancer Stat Facts [Internet]. SEER. [cited 2022 Apr 18]. Available online: https://seer.cancer.gov/statfacts/html/common.html
- Applewhite MK, James BC, Kaplan SP, et al. Quality of Life in Thyroid Cancer is Similar to That of Other Cancers with Worse Survival. World J Surg 2016;40:551-61. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19:1159-65. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Valderrabano P, McIver B. Evaluation and Management of Indeterminate Thyroid Nodules: The Revolution of Risk Stratification Beyond Cytological Diagnosis. Cancer Control 2017;24:1073274817729231. [Crossref] [PubMed]
- Endo M, Nabhan F, Porter K, et al. Afirma Gene Sequencing Classifier Compared with Gene Expression Classifier in Indeterminate Thyroid Nodules. Thyroid 2019;29:1115-24. [Crossref] [PubMed]
- Nikiforov YE, Baloch ZW. Clinical validation of the ThyroSeq v3 genomic classifier in thyroid nodules with indeterminate FNA cytology. Cancer Cytopathol 2019;127:225-30. [Crossref] [PubMed]
- Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol 2021;7:70-7. [Crossref] [PubMed]
- Nicholson KJ, Roberts MS, McCoy KL, et al. Molecular Testing Versus Diagnostic Lobectomy in Bethesda III/IV Thyroid Nodules: A Cost-Effectiveness Analysis. Thyroid 2019;29:1237-43. [Crossref] [PubMed]
- Wong CW, Schumm MA, Zhu CY, et al. Quality of Life Following Molecular Marker Testing for Indeterminate Thyroid Nodules. Endocr Pract 2020;26:960-6. [Crossref] [PubMed]
- Watt T, Bjorner JB, Groenvold M, et al. Development of a Short Version of the Thyroid-Related Patient-Reported Outcome ThyPRO. Thyroid 2015;25:1069-79. [Crossref] [PubMed]
- Schumm MA, Nguyen DT, Kim J, et al. Longitudinal Assessment of Quality of Life Following Molecular Testing for Indeterminate Thyroid Nodules. Ann Surg Oncol 2021;28:8872-81. [Crossref] [PubMed]
- Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018;124:1682-90. [Crossref] [PubMed]
- Patel KN, Angell TE, Babiarz J, et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg 2018;153:817-24. [Crossref] [PubMed]
- Jeon MJ, Lee YM, Sung TY, et al. Quality of Life in Patients with Papillary Thyroid Microcarcinoma Managed by Active Surveillance or Lobectomy: A Cross-Sectional Study. Thyroid 2019;29:956-62. [Crossref] [PubMed]
- Pitt SC, Saucke MC, Wendt EM, et al. Patients' Reaction to Diagnosis with Thyroid Cancer or an Indeterminate Thyroid Nodule. Thyroid 2021;31:580-8. [Crossref] [PubMed]
- Valderrabano P, Khazai L, Leon ME, et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocr Relat Cancer 2017;24:127-36. [Crossref] [PubMed]
- Vuong HG, Nguyen TPX, Hassell LA, et al. Diagnostic performances of the Afirma Gene Sequencing Classifier in comparison with the Gene Expression Classifier: A meta-analysis. Cancer Cytopathol 2021;129:182-9. [Crossref] [PubMed]
- Vuong HG, Ngo HTT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta-analysis. Cancer Cytopathol 2020;128:238-49. [Crossref] [PubMed]
Cite this article as: Sant VR, Livhits MJ. A narrative review of molecular testing for indeterminate thyroid nodules: living with the results. Ann Thyroid 2022;7:15.


