A narrative review of scars after surgery: what to expect
Introduction
Scars are an inevitable consequence of virtually every surgical procedure. While surgeons strive for minimally invasive or “scarless” surgery, these procedures typically decrease the size of scars or move them to less conspicuous areas rather than eliminating them completely. Some of these techniques are incredibly effective at scar camouflage—for example, the transoral vestibular approach for thyroid surgery hides the scar in the gingivobuccal sulcus, making it invisible in everyday interactions. However, not every patient’s anatomy and disease are amenable to this approach, and not every surgical procedure currently has an option for such minimally invasive access. Even with advances in surgical techniques, there will likely always be procedures that require placing scars in visible locations. For the best aesthetic outcome in these procedures, it is important to understand how to achieve the most cosmetically favorable scars possible. In this article, we explore normal wound healing and scar formation, factors that contribute to unfavorable scar formation, techniques to minimize the appearance of scars, and treatment options for unfavorable scars should they occur. The topics covered are applicable to all surgical and even some traumatic scars, but our figures and discussion focus on post thyroidectomy scars and their effect on patients’ quality of life. We present this article in accordance with the Narrative Review reporting checklist (available at https://aot.amegroups.com/article/view/10.21037/aot-22-16/rc).
Methods
A narrative review of the literature was conducted using our institution’s Primo search engine to search online databases including PubMed, EBSCO, and Ovid, among others. Included references were selected by the first author based on relevance to the subject matter and availability in English. The majority of articles reviewed were published within the last 15 years with two exceptions (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 3/16/2023 |
| Databases and other sources searched | Primo search engine used to search databases including PubMed, EBSCO, Ovid |
| Search terms used | Wound healing, hypertrophic scar, keloid, scar treatment, scar prevention, thyroidectomy scar |
| Timeframe | 1980–2023 |
| Inclusion criteria | All included studies were available in English; all selected studies were relevant to the review topic |
| Selection process | All sources selected by the first author |
Stages of wound healing
Hours to days after surgery
Wound healing begins immediately after surgery. The first stage of wound healing is hemostasis, which takes place in the first minutes to hours after surgery. Blood in the wound initiates the extrinsic clotting cascade and triggers the release of chemical signals that cause local vasoconstriction. Platelets aggregate on exposed subendothelial collagen and form a hemostatic plug at the site of injury. These platelets also secrete cytokines and growth factors which cause the release of scaffold proteins from surrounding cells. Scaffold proteins such as fibronectin, vitronectin, and thrombospondins contribute to a preliminary matrix for migrating keratinocytes, immune cells, and fibroblasts that will aid in wound healing and eventual scar formation. Platelet degranulation leads to release of inflammatory mediators, including interleukins (ILs) and tumor necrosis factor-alpha (TNF-α), and activates the complement cascade. Histamine released by the activated complement cascade causes capillary dilation and leakage, leading to accumulation of inflammatory cells in the wound bed and initiating the transition to the inflammatory phase of wound healing (1).
The inflammatory phase of wound healing takes place in the first 72 hours after surgery. During this phase, neutrophils and monocytes infiltrate into the wound bed (2). The majority of inflammatory cells present in the wound shortly after surgery are neutrophils. Neutrophils remove debris from the wound bed via phagocytosis and prevent infection at the surgical site during early wound healing. Neutrophils also phagocytose pathogens and destroy them with reactive oxygen species, proteases, and antimicrobial proteins. Neutrophil degranulation releases these compounds into the wound bed, causing further destruction of foreign organisms. Neutrophils regulate inflammation and release growth factors and cytokines that further wound healing by recruiting macrophages, T-cells, and additional neutrophils. After fulfilling these roles at the surgical site, neutrophils undergo apoptosis and are taken in by local macrophages, which triggers the transition out of the inflammatory phase. While neutrophils play a crucial role in early wound healing, persistent neutrophils in the wound bed are associated with delayed wound healing and chronic wounds. Proteases and reactive oxygen species released by neutrophils to eliminate pathogens in the wound bed can cause destruction of normal tissue if neutrophil presence is prolonged (1).
While these immune cells regulate wound healing internally, at the surface of the wound keratinocytes from the wound edge begin migrating towards the center. Keratinocytes at the surgical site release stored IL-1α, activating fibroblasts and adjacent keratinocytes. Fibroblasts secrete stimulating factors that further activate adjacent keratinocytes. Chemical signals from mast cells, monocytes, and macrophages in the healing wound help maintain activation of keratinocytes during re-epithelialization. Keratinocytes begin proliferating about a day after surgery and re-epithelialization should be complete within a few weeks. Keratinocyte activation should end once the wound is epithelialized. Prolonged activity of keratinocytes and fibroblasts after epithelialization is associated with hypertrophic scarring (3).
Days to weeks after surgery
Once the inflammatory phase of wound healing resolves, the surgical site enters the proliferation phase. This phase begins a few days after surgery and continues for several weeks. The proliferation phase consists of revascularization, re-epithelialization, and the generation of granulation tissue. Anti-inflammatory, pro-repair macrophages release chemical signals such as vascular endothelial growth factor (VEGF) that promote angiogenesis. Granulation tissue, composed predominantly of type III collagen and fibroblasts, forms in conjunction with these new blood vessels. Epithelial stem cells in the basal layer of the epidermis begin proliferating in response to chemical signals from pro-repair macrophages as well as fibroblasts in granulation tissue (1). As mentioned previously, epithelialization begins one day after surgery and should be complete within a few weeks. Wounds that fail to completely re-epithelialize within three weeks are more likely to form hypertrophied scars (3).
Weeks to months after surgery
Eventually, the granulation tissue in a healing wound transitions to scar in the remodeling phase of wound healing, which continues up to a year after surgery. Myofibroblasts develop from fibroblasts in the wound bed in response to mechanical tension as well as chemical signaling (1). Myofibroblasts cause contraction of the wound through their expression of smooth muscle actin. The type III collagen in granulation tissue is gradually replaced with stronger type I collagen (2). Myofibroblasts aid in this transition by releasing chemicals that degrade the weaker collagen of granulation tissue. Even once the transition to type I collage is complete, mature scars achieve only 80% of the tensile strength of intact, uninjured skin (1).
Excess inflammation in the wound throughout healing can lead to hypertrophic scar or keloid formation. Hypertrophic scars typically occur within 1–2 months of surgery. These cosmetically unfavorable scars often occur in areas of high tension and do not grow beyond the borders of the initial scar. Keloids can form at any time after surgery. In contrast with hypertrophic scars, keloids do extend beyond the border of the original scar. They do not typically have an association with high-tension wounds (1). It is important to consider the inflammatory reactions caused by various suture types and select a suture that causes minimal tissue reaction in patients who are prone to keloids or hypertrophic scars.
Preventative measures
In the early stages of wound healing, there are several interventions that can help prevent unfavorable scar appearance. It is important to achieve excellent hemostasis intraoperatively, as hematoma formation requiring reoperation leads to more trauma and tissue damage that could impact wound healing. Meticulous wound closure with atraumatic tissue handling and precise reapproximation and eversion of skin edges is critical for ideal scar appearance. It is important to strive for a closure without tension, as wounds healing under tension are more likely to form hypertrophic scars. Tethering of skin to underlying tissues is somewhat unique to thyroid surgery and can be prevented by a multi-layered closure with good soft tissue coverage of the trachea and cricoid cartilages.
Selection of suture material is another intraoperative factor that can play a role in the eventual appearance of scars. Natural sutures, such as plain surgical gut, fast-absorbing surgical gut, and chromic surgical gut, have been associated with inflammation and hypersensitivity reactions (4). Non-absorbable sutures such as Prolene and nylon have been shown to cause minimal cellular response in surrounding tissue and are considered inert (4). An in vitro study comparing Vicryl, Monocryl, nylon, silk, and several forms of polyester suture found that gene expression of pro-inflammatory markers was upregulated when exposed to silk, Vicryl, and polyester sutures (4). Monocryl and nylon sutures did not cause a significant change in gene expression of pro-inflammatory markers and had a similar response to negative controls (5). This data suggests that Monocryl, nylon, and Prolene sutures are good options to prevent excessive inflammation during wound healing. Avoidance of pro-inflammatory suture is particularly important in patients with a known history of keloids or hypertrophic scars.
Starting immediately after surgery, patients should be advised to avoid sun exposure to the operative site. Incisions should be kept clean and in a moist environment, which is best achieved with the application of ointment. Hydrophobic ointments such as Aquaphor are often recommended. Antibiotic ointments are preferred by some, though they can cause local dermatitis with extended use (6). The use of ointment during these initial phases allows quicker and improved re-epithelialization along the scar. Occlusive or semi-occlusive dressings can also be used in the immediate post-operative period, depending on surgeon preference.
Early intervention and continued prevention
As wounds complete re-epithelialization, several interventions can help prevent unfavorable appearance as scars continue to mature. As mentioned previously, patients should be counseled to avoid sun exposure to the area throughout the healing process. Patients should apply sunscreen or wear protective clothing if sun exposure to the area is unavoidable. In addition to routine wound care, silicone gel and pressure therapy are two options that can be used separately or in combination to help improve the appearance of scars.
Silicone gel
The application of silicone through silicone sheeting or topical silicone gel has been suggested to improve the appearance of scars in several studies and can also be used as a preventative measure (7,8). In a systematic review of randomized controlled trials evaluating silicone sheeting for the prevention and treatment of hypertrophic scars and keloids, 4 of 5 studies reviewed found that silicone sheeting as a preventative measure was associated with fewer abnormal scars, though the difference between groups was not statistically significant (7). A review of randomized controlled trials assessing silicone sheeting for the treatment of abnormal scars found that scar thickness, discoloration, and elasticity improved with the use of silicone sheets compared to no treatment. Of note, many of the studies reviewed had small sample sizes and subjective outcomes, limiting the strength of their results. Complications of silicone sheeting included transient rash and pruritus that resolved with removal of silicone sheeting (7).
Although it is not well understood exactly how silicone sheeting improves scar healing, it has been shown to reduce evaporation and improve scar hydration by more than 50%, which may contribute to improved scar appearance (8). Silicone is typically recommended for a minimum of 8 to 12 hours per day for 6 to 12 months. As there is little to no harm from the treatment and reasonable evidence of benefit, silicone sheeting should be considered for any scar that is healing with an unfavorable appearance (8). Topical silicone gels that include sun protection factor (SPF) are available and can be a convenient way for patients to get dual protection against cosmetically unfavorable scars.
Pressure dressing
Pressure therapy has been used for decades for thickened, hypertrophic scars and can be combined with silicone gel or sheeting. Pressure dressings come in many varieties and can include wrapped gauze or elastic dressings and adherent rigid or semi-rigid plates that apply pressure to the scar. On the neck, it is important that these apply pressure to the scar without causing discomfort to the patient as many structures in the neck are sensitive to compression. As with silicone sheeting, the exact mechanism by which pressure therapy improves scar appearance is unknown. It is postulated that by decreasing the supply of oxygen, blood, and nutrients to the scar, pressure therapy lowers collagen production in hypertrophied scars to that of normal scar tissue and encourages the realignment of existing collagen fibers to reduce scar thickness. Pressure therapy requires patience and can take 4 to 6 months to be effective. A study comparing silicone sheeting, pressure therapy, and combined silicone and pressure therapy found that all three treatments resulted in a significant improvement in scar thickness at 6 months, but this improvement was most significant with combined therapy (9). As with silicone sheeting, there is minimal potential for harm with pressure therapy, making it a reasonable option for patients with hypertrophic scars.
Treatment options for cosmetically unfavorable scars
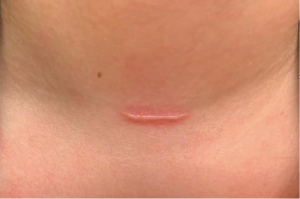
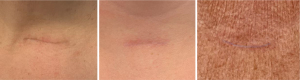
If scars have an undesirable appearance week to months after surgery, there are several interventions that can be offered to patients. At this time, scars may be noted to have irregular contour or may start to exhibit evidence of hypertrophy (Figure 1). There may also be lingering erythema or other dyspigmentation (Figure 2). Dermabrasion, laser resurfacing, and intralesional steroids can be helpful to address these problems. If these conservative measures are unsuccessful, surgical revision is a final option for improvement of scar appearance.
Dermabrasion
Dermabrasion involves mechanical removal of the outer layers of skin with a rapidly rotating device. It can be used to smooth out contour irregularities and reduce visibility of suture lines. This is ideally performed between 6 and 8 weeks after injury. It is beneficial to initiate treatment during this timeframe as there are more substantial improvements in appearance while the immature scar is still in the remodeling phase (10). Controlled ablation of the superficial skin can promote increased levels of hyaluronic acid at the incision site, which stimulates epidermal cells to migrate to the area and proliferate. Ideal candidates for dermabrasion have lighter complexions, as there is a risk of dyspigmentation in patients with darker complexions. Of note, it is recommended to wait 6–12 months before performing dermabrasion on patients who have taken 13 cis-retinoic acid (8).
Intralesional steroids
Intralesional corticosteroids, such as triamcinolone, can be used to improve the appearance of edematous scars, hypertrophic scars, and keloids. In edematous or “pin cushioned” scars, intralesional steroids can cause flattening and improved skin contour. Steroids should be injected into the dermis or the dermis-subcutaneous junction—if steroids are injected into the subcutaneous fat, they can cause atrophy of the fat resulting in a contour deformity (8). It is important to inject small doses as steroids can cause hypopigmentation and telangiectasias if higher concentrations are used. One study suggests injecting 1 mg (0.1 mL of 10 mg/mL triamcinolone) in multiple sites at least 1 cm apart. The total injected dose should be limited to 30–40 mg to avoid adverse effects. Intralesional corticosteroid injections can be repeated every 4 to 6 weeks (11). Patients should be monitored for adverse effects of steroid injections including fat atrophy, hypopigmentation, and telangiectasias while receiving treatment.
Laser resurfacing
Laser resurfacing can be used to address irregular contour of scars as well as dyspigmentation. Lasers cause microscopic thermal injury in the skin which triggers tissue remodeling. Three types of lasers are beneficial for scar revision—pulsed-dye lasers (PDL) and similar lasers that target small blood vessels, Nd:YAG lasers, and ablative and non-ablative fractional lasers.
Pulsed-dye lasers cause selective photothermolysis of small blood vessels. Potassium titanyl phosphate (KTP) lasers are similar to pulsed-dye lasers and also target small blood vessels. Nd:YAG lasers cause selective photothermolysis of microvessels and pigmented cells. Because these lasers target blood vessels and pigmented cells, they are ideal for treatment of erythematous scars. They may be effective as monotherapy for small hypertrophic scars, but can also be combined with fractional lasers in concurrent or alternating sessions for scars with more severe hypertrophy. Pulsed-dye laser treatments can be repeated every 1 to 2 months. Treatment with these lasers also seems to be effective at relieving the itching associated with inflamed, erythematous scars (12).
Ablative and non-ablative fractional lasers are non-selective and cause microscopic thermal damage throughout the dermis and epidermis. Fractional lasers are much more effective per treatment at improving scar appearance than PDL or Nd:YAG lasers, and ablative fractional lasers are more effective than non-ablative. Fractional lasers are best suited for hypertrophic or contracted scars. Ablative fractional lasers include carbon dioxide (CO2) lasers and erbium:YAG lasers. These lasers have greater depth of penetration and are therefore ideal for treating thicker, hypertrophic scars. Treatment depth should be tailored to scar thickness and should not exceed the thickness of the scar. Treatment with ablative fractional lasers can be repeated at intervals of 2–3 months. Patients often need multiple treatments to achieve the best results, with a series of 3 to 6 treatments being common. It is important to avoid excessive thermal injury to the area to prevent potential worsening of scarring. This is done by using a narrow beam diameter, short pulse width, and minimizing the number of passes over the area (12).
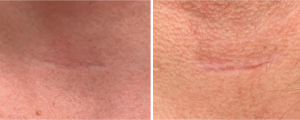
Non-ablative fractional lasers are well-suited for treating atrophic scars (Figure 3). Atrophic scars are flat or depressed due to decreased collagen, and laser treatment can help stimulate collagen deposition in the area. Non-ablative lasers can be as effective as ablative lasers at promoting collagen synthesis and are generally better tolerated. However, non-ablative lasers may require more treatments to achieve equivalent results to ablative lasers (12).
As with dermabrasion, scars can be treated with laser resurfacing during the remodeling phase at 6–8 weeks post-injury to reduce the appearance of scars while they are forming (10). Studies have shown that early treatment with pulsed-dye lasers in the first few weeks after surgery resulted in earlier resolution of scar stiffness and erythema and decreased formation of hypertrophic scars (10). There is some evidence that treatment of healing wounds with unstable epidermal coverage in the first 1 to 3 months can potentially be harmful. However, epithelialized wounds that are relatively mature can be safely treated with lasers (12).
Surgical revision
If scars have persistent hypertrophy or dyspigmentation despite these interventions, surgical scar revision is available as a final option. If a patient develops steroid-resistant keloids, surgical excision should be performed via an elliptical incision around the keloid with primary closure. Intralesional steroids or topical imiquimod can be used to prevent recurrence of the keloid. For recalcitrant keloids, radiation can also be used. For hypertrophic or aesthetically displeasing scars, scar revision can be performed. Scars should be fully matured, at least 6–12 months after the initial surgery, prior to any revision procedures. Techniques include serial excision as well as realignment of the scar with a Z-plasty, W-plasty, or geometric broken line techniques. These techniques can have the effect of lengthening the scar, but also serve to reorient the scar so that it is less visible or closer to resting skin tension lines (8).
Discussion
While surgeons should always strive for excellent cosmesis after surgery, it would be naïve to dismiss postsurgical scar appearance as a purely cosmetic issue. Unfavorable scars can have a significant impact on patients’ quality of life, particularly when they are located on highly visible areas like the anterior neck, as is the case with thyroid surgery. Several studies have investigated the impact of a cosmetically unfavorable scar on patients’ quality of life (13-16). Brown et al. conducted one-on-one interviews with 34 patients attending a plastic surgery clinic for management of unfavorable scars (13). Roughly half of these patients reported feeling stigmatized by their scars. Some patients expressed fear that others would think their scars were self-inflicted. Half of the patients felt their personal relationships had suffered as a result of their scars, and one-third felt less social and avoided public situations (13). Of the patients who were employed, three-quarters reported hiding their scars during job interviews (13). This study focuses on a biased population, as all patients interviewed were seeking treatment with a scar revision specialist. However, other studies have shown scars can impact patients’ quality of life even if there is only minimal hypertrophy or dyspigmentation.
In 2014, a study looking specifically at the effect of post-thyroidectomy scars on quality of life was conducted using the Dermatology Life Quality Index (DLQI), which has previously been used to evaluate quality of life in patients with chronic skin conditions. Ninety-seven patients were included in the study, of which 64 (66%) reported physical symptoms including tightness, pruritis, burning, or pain related to their scar. The main areas in which patients reported impact on their quality of life were choice of clothing and participation in social and leisure activities. Patients also reported feeling self-conscious or embarrassed by their scar. One important characteristic of this study is that it did not focus exclusively on patients with severely disfiguring scars—patients included in this study had a wide range of scar appearances. The Vancouver scar scale (VSS) was used to score the post-thyroidectomy scars of all participants and patients were divided into four categories—32 patients (33%) had a flat linear scar, 9 (9.3%) had a raised linear scar, 41 (42.3%) had a hypertrophic scar, and 15 (15.5%) had an adherent or tethered scar (14). None of the patients included were described as having keloids. The Dermatology Life Quality Index did not vary significantly among the different scar types included or with the patients’ VSS scores, which suggests that even mildly abnormal scars can have an impact on patients’ quality of life (14). This was particularly true if patients’ had physical symptoms such as pruritis or pain associated with their scar (14).
Some studies have compared the impact of scars to chronic medical conditions to validate the effect they have on a patient’s quality of life. Quality of life impairment in patients with hypertrophic scars and keloids has been found to be similar to that of patients with chronic dermatologic conditions like psoriasis and severe atopic dermatitis (15). Previous studies have shown that patients with chronic skin conditions can experience the same reduction in quality of life as patients with life-threatening medical comorbidities, such as congestive heart failure (16). Clearly, postsurgical scars can have a serious impact on patients’ quality of life and cannot be dismissed as an aesthetic issue alone.
Further evidence of the impact that an anterior neck scar can have on quality of life can be found in studies comparing traditional transcervical thyroidectomy and transoral thyroidectomy, which is virtually scarless. A prospective study comparing quality of life scores from 61 patients undergoing transcervical thyroidectomy and 60 patients undergoing transoral thyroidectomy found that cosmetic outcome and overall satisfaction were significantly higher in the transoral group (17). Transcervical thyroidectomy patients also reported more concerns with physical and emotional restrictions on their daily activities compared with transoral thyroidectomy patients (17). While transoral thyroidectomy is scarless and eliminates the quality of life impact that scars can have, not every patient is a good candidate for this approach, and not every surgeon has adequate training in this approach. For this reason, it is still crucial to understand and apply techniques to improve the appearance of scars.
Despite careful intraoperative technique and good wound management postoperatively, some patients still heal with unfavorable scars. While there are many good options to improve the appearance of scars after surgery, it can be challenging to identify what methods to use first and when to initiate treatment. The International Clinical Recommendations on Scar Management proposes an algorithm for scar prevention and treatment that can be helpful.
For prevention of scars, they recommend careful surgical technique and good wound care for all patients, including protection from sun exposure. Patients with a history of hypertrophic or keloid scarring are considered high risk and are recommended silicone gel or sheeting after the wound has epithelialized with consideration of concurrent intralesional corticosteroids. Patients at low risk for hypertrophic scar or keloid formation can be recommended silicone gel or sheeting if they express concerns about scarring, but otherwise do not require any preventative intervention (18).
For treatment of cosmetically unfavorable scars, recommendations varied based on scar type. Patients with immature hypertrophic scars that are red and slightly raised should be recommended silicone gel or sheeting. If there is no improvement after one month, these scars can be treated as mature linear hypertrophic scars. Alternatively, immature hypertrophic scars can be treated with pulsed dye laser therapy monthly for 2–3 months before advancing to the mature hypertrophic scar algorithm. Mature linear hypertrophic scars can be treated with silicone gel or sheeting for 2 months before progressing to intralesional corticosteroid or 5-fluorouracil injections, which can be repeated monthly. For linear hypertrophic scars that do not resolve with these first-line treatments, pulsed-dye or fractional laser therapy are additional options. Laser treatment can also be combined with pressure therapy. If there is no significant improvement after a 12-month period of treatment, surgical scar revision is a reasonable consideration. Surgical scar revision should be considered earlier in wounds that are clearly under tension. If there is no obvious tension contributing to the poor wound healing, it is reasonable to continue conservative management for 1 year prior to offering scar revision (18).
Keloids are physiologically distinct from hypertrophic scars and have a slightly different treatment algorithm. Per the algorithm, first-line therapy for minor keloids includes silicone gel combined with monthly intralesional corticosteroid injections. If there is no improvement in 8–12 weeks, laser therapy or surgical excision can be considered. Silicone gel and intralesional corticosteroids can be used prophylactically to reduce recurrence of keloid after surgical excision. For major keloids, monthly intralesional corticosteroids injections are first-line management. If this treatment is not effective after 3–4 months, intralesional 5-fluorouracil can be used in addition to triamcinolone. Secondary options include laser treatment and surgical excision. Postoperative steroids and radiation therapy can be considered in severe or recurrent keloids. Referral to a specialist experienced in keloid management is warranted for these complicated cases (18).
Conclusions
Despite advances in minimally invasive approaches, scars remain an inevitable consequence of most surgical procedures. Because scars with unfavorable appearances are known to affect a patient’s quality of life, it is important to strive for scars that are well camouflaged with the surrounding skin. Preventative measures in the intraoperative and early postoperative period can help reduce the risk of poor scar formation, and many treatment options exist should a scar develop hypertrophy, dyspigmentation, or pruritis. Thyroid cancer patients have an overall excellent post-treatment prognosis and generally are able to lead long and active lives after thyroidectomy. Therefore, given the potential for negative impact on quality of life especially over the course of many years, it is important not to dismiss a patient’s concerns about their scar, and to recognize when to refer them to an expert if initial conservative measures are unsuccessful.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Thyroid for the series “Improved Quality of Life after Thyroid Surgery”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aot.amegroups.com/article/view/10.21037/aot-22-16/rc
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-22-16/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-22-16/coif). The series “Improved Quality of Life after Thyroid Surgery” was commissioned by the editorial office without any funding or sponsorship. EC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ellis S, Lin EJ, Tartar D. Immunology of Wound Healing. Curr Dermatol Rep 2018;7:350-8. [Crossref] [PubMed]
- Mony MP, Harmon KA, Hess R, et al. An Updated Review of Hypertrophic Scarring. Cells 2023;12:678. [Crossref] [PubMed]
- van der Veer WM, Bloemen MC, Ulrich MM, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns 2009;35:15-29. [Crossref] [PubMed]
- Salthouse TN. Biologic response to sutures. Otolaryngol Head Neck Surg (1979) 1980;88:658-64. [Crossref] [PubMed]
- Lock AM, Gao R, Naot D, et al. Induction of immune gene expression and inflammatory mediator release by commonly used surgical suture materials: an experimental in vitro study. Patient Saf Surg 2017;11:16. [Crossref] [PubMed]
- Draelos ZD, Rizer RL, Trookman NS. A comparison of postprocedural wound care treatments: do antibiotic-based ointments improve outcomes? J Am Acad Dermatol 2011;64:S23-9. [Crossref] [PubMed]
- O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013;2013:CD003826. [Crossref] [PubMed]
- Thomas JR, Mosby SR. Scar revision and camouflage. In: Flint PW, Haughey BH, Lund VJ, et al, eds. Cummings Otolaryngology - Head and Neck Surgery. Philadelphia, PA: Elsevier Mosby; 2010:295-301.
- Li-Tsang CW, Zheng YP, Lau JC. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res 2010;31:448-57. [Crossref] [PubMed]
- Oliaei S, Nelson JS, Fitzpatrick R, et al. Laser treatment of scars. Facial Plast Surg 2012;28:518-24. [Crossref] [PubMed]
- Bloemen MC, van der Veer WM, Ulrich MM, et al. Prevention and curative management of hypertrophic scar formation. Burns 2009;35:463-75. [Crossref] [PubMed]
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol 2014;150:187-93. [Crossref] [PubMed]
- Brown BC, McKenna SP, Siddhi K, et al. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 2008;61:1049-58. [Crossref] [PubMed]
- Choi Y, Lee JH, Kim YH, et al. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann Dermatol 2014;26:693-9. [Crossref] [PubMed]
- Balci DD, Inandi T, Dogramaci CA, et al. DLQI scores in patients with keloids and hypertrophic scars: a prospective case control study. J Dtsch Dermatol Ges 2009;7:688-92. [Crossref] [PubMed]
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999;41:401-7. [Crossref] [PubMed]
- Xuan Nguyen H, Nguyen HX, Thi Hoang H, et al. Quality of Life and Surgical Outcome of Transoral Endoscopic Thyroidectomy Vestibular Approach (TOETVA) versus Open Thyroid Surgery: Experience from a Single Center in Vietnam. J Thyroid Res 2022;2022:2381063. [Crossref] [PubMed]
- Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatol Surg 2014;40:825-31. [PubMed]
Cite this article as: Landers K, Hwang M, Cottrill E. A narrative review of scars after surgery: what to expect. Ann Thyroid 2023;8:9.